![]()
Titration of a Polyprotic Acid
Introduction
Two important concepts in chemistry are titration and acid-base reactions. Titration is the method of determining the concentration of a solution by allowing a carefully measured volume of a substance to react with a standard solution of another substance, whose concentration is known. By adding a carefully measured volume of a base of known concentration to a carefully measured volume of acid of unknown concentration, or vice versa, and using your knowledge of stoichiometry and acid-base neutralization, you can determine the concentration of the unknown. In today’s experiment, you will use this process to titrate an unknown sample of a triprotic acid and determine its concentration. |
Background
While this investigation focuses upon acid-base chemistry and the use of titration to determine concentration, titration as an analytical method has many applications. In general terms, titrations utilize a known property of one solution to determine a similar property of an unknown solution. Specifically, these include acid-base titrations, potentiometric titrations (redox), complexometric (formation of a colored complex) titrations, and even titrations utilized to determine specific concentrations of bacteria or viruses. For example, the alkalinity and acidity of water in streams and rivers is an important topic to environmental chemists. In order to determine such characteristics, they use the same technique you will learn in this experiment— acid-base titration.
Titration Set-up
The generalized setup of a titration is shown here:
 |
Picture depicts a Buret, Ring Stand, and Buret Clamp (orange) |
The base is placed in the buret, so that a precise amount of solution can be added to the acid. The buret's precision is attributed to the graduations on the tube, making it one of the more expensive pieces of glassware in the lab. The precision of the buret is dependent upon reading it correctly: volumes delivered by a buret are read to the hundredth of a milliliter . The knob on the buret is called a stopcock, and its sole purpose is to deliver the titrant to the solution below in a controlled manner.
Acid-Base Titrations
When an acid solution is titrated with a strong base such as NaOH, the initial pH of the solution is low. As base is added to the acidic solution, the pH gradually rises until the volume added is near the equivalence point, the point during the titration when equal molar amounts of acid and base have been mixed. Immediately before the equivalence point, the pH increases very rapidly and then levels off again immediately after the equivalence point with the addition of excess base (Figure 2). The equivalence point is located in the center of the vertical portion of this line. In this experiment, a carefully measured volume of unknown acid is titrated with NaOH of known concentration. Since the buret allows us to determine the precise amount of base needed for neutralization, the precise concentration of the acid can be calculated.
|
Titration Technique |
Visualizing the ‘end’ of a particular titration, specifically referred to as the endpoint or equivalence point, is essential to a successful titration. Indicators, often added in minute amounts to the solution of interest, are chemical compounds that undergo dramatic changes of color when a particular property of a solution is changed. Indicators are specific to the reaction being analyzed. The endpoint is the point in the titration where the indicator changes color and the equivalence point is the point in the titration when the stoichiometric amount of titrant has been added and the moles of acid and base are equal. An indicator is generally chosen so that endpoint is roughly equivalent to the equivalence point.
pH and pH Meters
 |
The hydrogen ion concentration, expressed in terms of pH, is one of the most important properties of aqueous solutions, as it can control the solubility of various species, the formation of complexes, and even the kinetics of an individual reaction. In order to obtain precise data of the particular hydronium concentrations of the solutions in this experiment, and to clearly observe the change in pH at the equivalence point, a pH meter is used. In general, a pH meter measures the differences in electromotive force between two electrodes. A pH meter contains an electrode sensitive to the concentration of the hydrogen ion as well as one used solely for a reference. For accurate measurements, it is necessary to calibrate the instrument using a buffer solution of approximately the same pH as the sample to be used. This calibration takes care of temperature effects and minor variations in the potential due to changes in the membrane. Your instructor will provide details regarding the calibration of the pH meters used in your laboratory. |
Weak Acid Equilibria
A weak acid (HA) is one that does not fully dissociate in water. In other words, if the weak acid represented is allowed to ionize, as shown in the equation below, then a significant amount of HA will remain un-ionized.
HA(aq) + H2O(l) ![]() H3O+(aq) + A-(aq)
H3O+(aq) + A-(aq)
At equilibrium, the dissociation of a weak acid is generally described by its acid-dissociation constant (Ka) and is mathematically represented as follows:
![]()
In this investigation the acid-dissociation constant of an unknown triprotic acid is experimentally determined. With the knowledge that at equilibrium the concentration of the free hydronium ions (H3O+) is equal to the concentration of the conjugate base (A¯), if the concentration of either of these chemicals is determined experimentally, then stoichiometry can be used to determine the concentrations of the other components in the solution. Mathematically, the relationship for the reaction above is expressed as:
[HA]Eq = [HA]Init – [H3O+]Eq = [HA]Init – [A¯]Eq
From this logic, combined with the fact that pH is equal to the negative log of the hydrogen ion concentration, we can arrive at an expression for Kaincorporating only the initial concentration of the weak acid, and the experimentally determined pH at the equivalence point.
![]()
The acid-dissociation constant of a weak acid can also be determined by another method. This method involves the ‘half equivalence point’, where just enough NaOH has been added to the weak acid to convert half of the acid to its salt. At this point, the concentration of the weak acid, [HA], is equal to the concentration of its conjugate base, [A¯]. Utilizing this fact, our generalized equilibrium expression equation (1) can now be defined as shown below because [A¯] and [HA] can be canceled out of the expression.
At ½ Equivalence: Ka= [H3O+] and pKa = pH
Overall, by performing these titrations and plotting the pH versus volume of NaOH added, you can see how the pH of the solution changes as an acid or base is added.
Pre-Laboratory Question 6: If the pH at one-half the first and second equivalence points of a diprotic acid is 3.5 and 6.2, respectively, what are the values for pKa1 and pKa2 and Ka1 and Ka2? Items in red should be variable. pKa1 pKa2 Ka1 Ka2 (SUBMIT) |
Polyprotic Acids
Triprotic acids have three ionizable hydrogens and thus three separate pKa values, one for each dissociation. At any point along the titration curve of a triprotinc acid, there is some percentage of each acid form present in the mixture. This means that unlike a monoprotic dissociation that is “all or nothing,” the pH of a polyprotic acid solution is dependent on several forms of the acid. The figure below depicts the generalized percent dissociation of a triprotic acid as it is being titrated with base. Note that even as the third proton has started to dissociate some H3A is still present in the system.
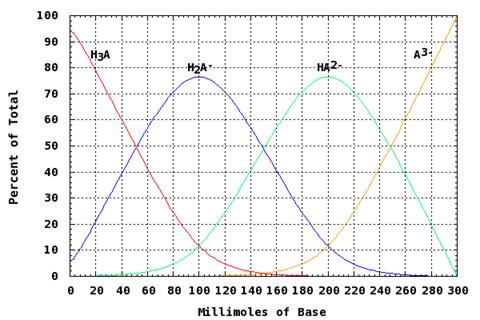
Figure 3: Percent Dissociation of a Triprotic Acid During Titration
This also means that more than one inflection point is observed in the titration curves. Figure 4 below shows the titration curve of phosphoric acid. Note that although there are three pKa values, the third equivalence point is not shown. In order for the titration to reveal that point, the pH of the base used would need to far exceed the value of the third pKa. The third pKa value for phosphoric acid is 12.4. The base used in the titration would have to exceed this value by about 2 pH units to produce the third equivalence point. NaOH at titration concentrations (0.1M – 0.5M) has a maximum pH of about 13 and therefore the third equivalence point is not shown.
Pre-Laboratory Question 7: Complete the Table below for use during your lab experiment
(SUBMIT) (NOTE: This table will be made available in your procedure and report sections for reference) (SUBMIT) |

Figure 4: Titration Curve of Phosphoric Acid with Strong Base
Pre-Laboratory Question 1: A triprotic acid requires three moles of base to neutralize it, and the protons are removed one at a time as follows: As an example, you have a 40.0 mL solution of a triprotic acid, H3A, with a concentration of 0.0588 M. You titrate it with a 0.250 M solution of NaOH. How many moles of H+ are you titrating? (Give units). Items in red should be variable. Moles H+ (SUBMIT) |
Pre-Laboratory Question 2: How many moles of NaOH are required to complete the titration in Question 1? (Give units). Moles NaOH (SUBMIT) |
Pre-Laboratory Question 3: What volume of NaOH will be needed to completely titrate the acid in Question 1? Vol NaOH (SUBMIT) |
Pre-Laboratory Question 4: What volume of NaOH will be needed to reach the first equivalence point in Question 1? Vol NaOH (SUBMIT) |
Pre-Laboratory Question 5: What volume of NaOH will be needed to reach the second equivalence point in Question 1? Vol NaOH (SUBMIT) |