Energy
The content that follows is the substance of General Chemistry Lecture 21. In this lecture we cover the definitions of Energy and the process of Calorimetry.
Energy
Unfortunately to have a discussion about the topics that follow there are a great deal of definitions that must be discussed first so bear with me.
First and foremost we need to define this section of study in chemistry. Thermodynamics or more specifically Thermochemistry is the study of the absorption or release of heat that accompanies chemical reactions.
Energy is defined as the capacity to do work and and in chemistry we define Energy as the sum of both the work (w) done and the heat (q) generated or lost.
E = w + q
Energy also falls into two general types:
(1) Kinetic Energy (EK)or the energy of motion: 1/2 mv2 where m is mass and v is velocity
(2) Potential Energy(EP): the energy stored in chemical bonds that is released when a bond is broken or formed.
In the picture below, as the water begins to flow, the energy changes from potential to kinetic:

In any case, the energy measured is generally expressed in one of two units: Calories (cal) or Joules (J) A Joule is defined as 1kg.m2/s2. A calorie is the amount of heat required to raise 1 mole of water by 1 degree Celsius and a 4.184 J = 1 cal.
Law of Conservation of Energy
Just like the Law of Conservation of Mass set the stage for stoichiometric calculations, the Law of Conservation of Energy sets the stage for thermodynamic calculations.
The law states that Energy can neither be created nor destroyed and is therefore known as the First Law of Thermodynamices. This means that the total Energy of the universe is constant and only changes in type of energy can be measured.
In chemistry this often means that the potential energy found in chemical bonds is converted to heat energy which in turn is then converted into kinetic energy as molecule respond to the increase in temperature etc. Or as shown below the formation of a bond converts chemical energy into heat and light energy. The important feature of the process is that the amount of energy remains constant.
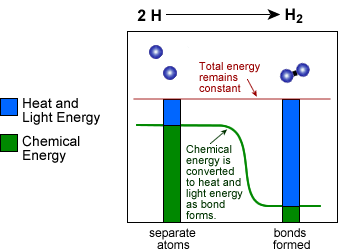
Here are a couple more definitions for you:
1) Temperature - in thermodynamics this is defined as a measure of kinetic energy derived from molecular motion. Kind of a which came first Chicken or Egg kind of definition since at higher temperatures molecules have greater kinetic energy but there you have it.
2) Thermal Energy or Heat (q) - is determined as the kinetic energy transferred from one object to another as a result of a temperature difference between them. So we have to measure the difference in temperature between two objects/substances to determine the heat.
3) Chemical Energy - as shown above this is the potential energy found in chemical bonds.
Energy Calculations
At this point there is only a couple of types of energy calculations we can make Energy from work and heat.
For example:
What is the energy of a system that does 100 J of work and absorbs 250 J of heat?
In order to calculate this you need to know the sign conventions:
In thermodynamics (much like in life) doing work is given a negative sign and having work done on the system is positive. In the same way, losing heat is given a negative sign and absorbing heat is a positive. So for the problem above it is really just a reading interpretation process since the math involved is just addition or subtraction:
The system is doing work so the 100 J should be negative and it is absorbing heat so the 250J is positive:
E = -100J + 250J = 150 J
and the other type of calculation is for kinetic energy:
EK= 1/2 mv2
So here is a simple example:
If a linebacker can run 40 meters in 4.15 seconds and weighs 195 lbs how much kinetic energy does he have?
First remember the units of a Joule: 1kg.m2/s2
We need to convert the lbs to kg: 195 lbs x 1 kg/2.2lb = 88.6 kg
Then it is just plug and chug:
EK = ½(88.6kg)(40m/4.15s)2 = 4.12 kJ
And now back to definitions...
System and Surroundings
As we learned earlier, the energy lost and gained must be equivalent for any process, but we didn't define where we were going to lose or gain this energy so we will do that now.
The System - is defined as the process, reaction or object under study.
The Surroundings - is everything else.
For example in a reaction of an aqueous acid and base, the system would be the reaction itself between the acid and base molecules and the surroundings would be the water and container they are in.
Officially there are three types of systems:
Open - Can exchange heat and matter with the surroundings
Closed - Can exchange heat but not matter with the surroundings
Isolated - Cannot exchange heat or matter with the surroundings
At this point I want to remind you again of the First Law of Thermodynamics: Energy is neither created nor destroyed. Another way of stating this is that the net Change in Energy between a system and its surroundings must be zero. ΔE = ESystem + ESurroundings=0. In order for this to be true, then the Energy of the system must be equivalent to the energy of the surroundings but opposite in sign.
ESystem = -ESurroundings or -ESystem = ESurroundings

And just like before, when the system is losing energy, we give it a negative sign and when it is gaining energy it is a positive.
Heat
When a chemical reaction occurs in an open container most of the energy gained or lost is in the form of heat. Almost no work is done (i.e. nothing is being moved). Heat(q) is defined as an energy transfer between a system and its surroundings. Heat flows between the system and surroundings until the two are at the same temperature. When a chemical reaction occurs in which the system absorbs heat, the process is said to be endothermic (it feels cold). When a chemical reaction occurs in which the system produces heat it is exothermic (it feels hot).
Determination of Heat - Calorimetry
Calorimetry is a process by which the change in temperature of a system is measured.
There are two basic types of calorimetry - Constant Pressure and Constant Volume.
A Constant Pressure Calorimeter is often called a coffee cup calorimeter because it resembles and is often constructed of a coffee cup in laboratory experiments.
 |
 |
The qSys shown here is the total heat for the entire calorimeter which contains the water and calorimeter of the surroundings as we have defined it previously and the qRxn we defined as the system previously. Just as above where we stated the overall energy had to equal to zero so does the qSys shown here. This means that the rearrangement of the equation above sets the q of the surroundings equal in magnitude but opposite in sign just as before.
Calculating q is dependent on not only the change in temperature but also on its specific heat capacity and amount.
Specific Heat Capacity (c)
The specific heat (c) of a substance is the amount of heat (q) required to raise the temperature of one gram of the substance by one degree Celsius.
 |
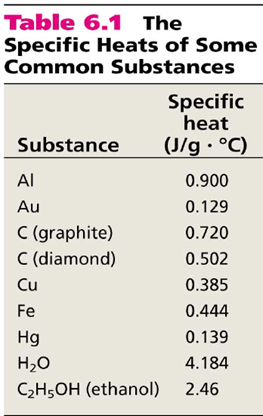 |
This equation for heat just means that the more of a substance you have, the more heat it can absorb or release so heat is considered an extensive property. And it also takes into consideration that different types of materials absorb/release heat differently. Anyone that has ever cooked with aluminum foil or a cast iron pan understands this difference. The foil you can touch with minimal danger as it is cool almost immediately after removal from heat but an iron pan will remain dangerous to touch for a long time. This is because of their different heat capacities (see table above).
Getting back to calorimetry, the other type of calorimeter is a constant volume or "bomb" calorimeter. The "bomb" part comes from the fact that since the volume is constant the pressure inside the calorimeter can be quite high. If not watched carefully this can lead to disasterous results.
 |
 |
The equations for the calculation of heat are virtually identical to those of the constant pressure calorimeter. The big difference between the two is that while in the coffee cup calorimeter the reaction takes place in the water as part of the aqueous solution, the reaction in a bomb calorimeter is in a separate conpartment altogether.
Here are some simple calorimetry examples: