Hybridization
The content that follows is the substance of General Chemistry Lecture 35. In this lecture we Introduce the concepts of valence bonding and hybridization.
Valence Bond Theory
The Valence Bond Theory is the first of two theories that is used to describe how atoms form bonds in molecules. In this theory we are strictly talking about covalent bonds.

According to the theory, covalent (shared electron ) bonds form between the electrons in the valence orbitals of an atom by overlapping those orbitals with the valence orbitals of another atom.
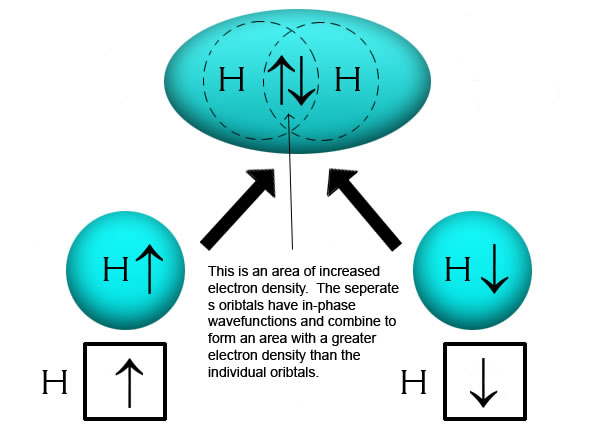
When the bonds form, it increases the probability of finding the electrons in the space between the two nuclei.
There are two different types of overlaps that occur: Sigma (σ) and Pi (π)
Sigma (σ) Bonds form between the two nuclei as shown above with the majority of the electron density forming in a straight line between the two nuclei. I often refer to this as a "head-to-head" bond.
Pi (π) Bonds form when two un-hybridized p-orbitals overlap. This is what I call a "side-by-side" bond.

Pi (π) Bond
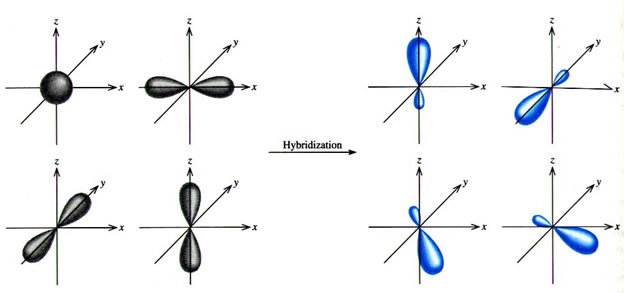
In order to overlap, the orbitals must match each other in energy. The process by which all of the bonding orbitals become the same in energy and bond length is called hybridization.
Hybridization
Let's start this discussion by talking about why we need the energy of the orbitals to be the same to overlap properly.
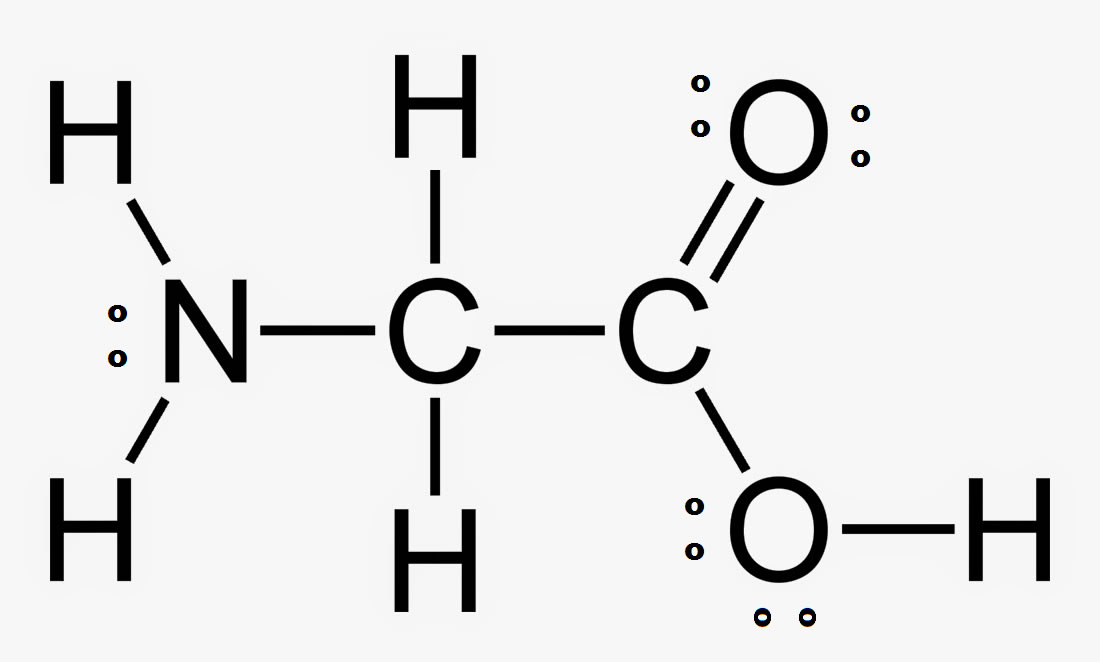
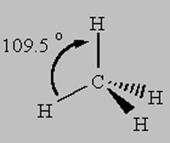
Let's look at the bonds in Methane, CH4
The Carbon in methane has the electron configuration of 1s22s22p2. According to Valence Bond Theory, the electrons found in the outermost (valence) shell are the ones we will use for bonding overlaps. This will be the 2s and 2p electrons for carbon.
As you know, p electrons are of higher energy than s electrons. This means that the two p electrons will make shorter, stronger bonds than the two s electrons right? But this is not what we see. We see a methane with four equal length and strength bonds. So how do we explain this? Simple: Hybridization
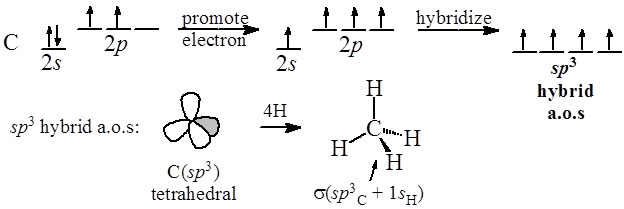
One of the s orbital electrons is promoted to the open p orbital slot in the carbon electron configuration and then all four of the orbitals become "hybridized" to a uniform energy level as 1s + 3p = 4 sp3 hybrid orbitals.
Identifying Hybridization in Molecules
Figuring out what the hybridization is in a molecule seems like it would be a difficult process but in actuality is quite simple. Because hybridiztion is used to make atomic overlaps, knowledge of the number and types of overlaps an atom makes allows us to determine the degree of hybridization it has. In other words, you only have to count the number of bonds or lone pairs of electrons around a central atom to determine its hybridization.
The following rules give the hybridization of the central atom:
1 bond to another atom or lone pair = s (not really hybridized)
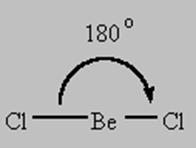
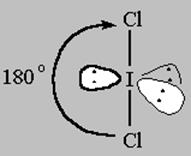
2 bonds to another atom or lone pairs = sp
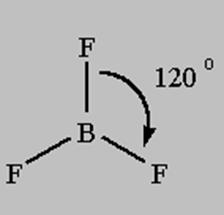
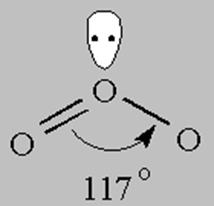
3 bonds to another atom or lone pairs = sp2
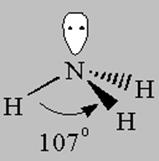
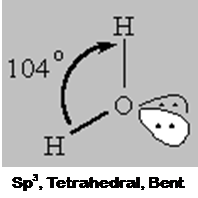
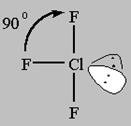
4 bonds to another atom or lone pairs = sp3
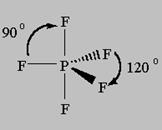
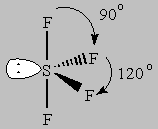
5 bonds to another atom or lone pairs = sp3d
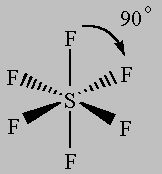
6 bonds to another atom or lone pairs = sp3d2This Video Explains it further: