Ionic Formulas and Nomenclature
This is the 6th lecture for General Chemistry. The topics covered in this lecture are Ions, Ionic and Molecular (Covalent) Compounds, Polyatomic Ions and Ionic Nomenclature.
Ions
What is an ion? An ion is an element whose proton and electron counts are not equal. If an element has more protons than electrons it will be positively charged and if the element has more electrons than protons it will be negatively charged. Ions form naturally based on where they are located in the periodic table.
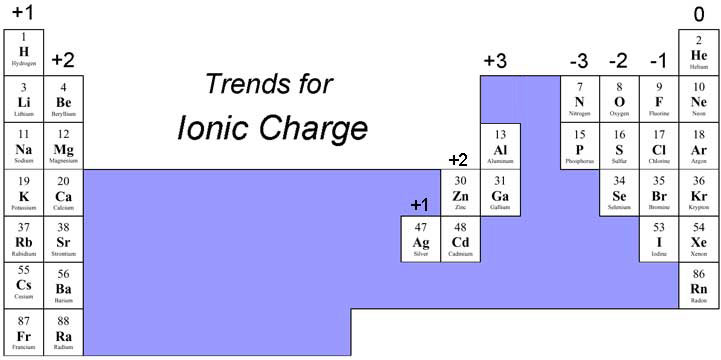
As you can see from the partial table shown above the Groups of the periodic table each form a unique charge of ion. The Natural formation of ions is:
Group 1 elements form +1 ions
Group 2 elements form +2 ions
Group 13 elements form +3 ions
Group 14 elements form none
Group 15 elements form -3 ions
Group 16 elements form -2 ions
Group 17 elements form -1 ions
Group 18 elements form none.
One of the things you should really notice in this listing is that Metals form Positive ions and Non-metals form negative ions.
Another thing you should immediately notice is that we skipped over the Transition Metal groups in our listings. This is because the transition metals can often form more than one type of ion:

Let's Practice:
Molecular versus Ionic Compounds
It was remarked in the previous lecture that knowing which elements are metals and which are non-metals is very important. Now we will explain why. When metals are combined with non-metals to form compounds they do so by charge attraction. This means that positive ions are attracted to negative ions and they add to one another until the charge is neutralized. This would be defined as an Ionic Compound.
For example Sodium (Na) forms a +1 ion and Sulfur (S) forms a -2 ion. If they got together to form a molecule, there would have to be 2 sodium ions for every 1 sulfur ion to make a neutral molecule. This molecule would be written symbolically as Na2S and called Sodium Sulfide.
The math for this combination is very simple 2(+1) + (-2) = 0
When two non-metals form a bond, they do so by sharing electrons. This process will be covered in detail in a later lecture. For now, you simply need to be able to determine whether a molecule is covalent (molecular) or ionic in nature since the two types of compounds have very different naming rules (nomenclature). This is why it is so important for you to know the locations of the metals and non-metals in the periodic table.
Polyatomic Ions
A very special category of ions are called polyatomic ions and this is because they contain more than one element but a single charge. Generally these are non-metals in a covalent bonded system that when combined have more or less electrons than protons thus leading to a charge on the overall molecule. There are tables of polyatomic ions listed in your book and below.
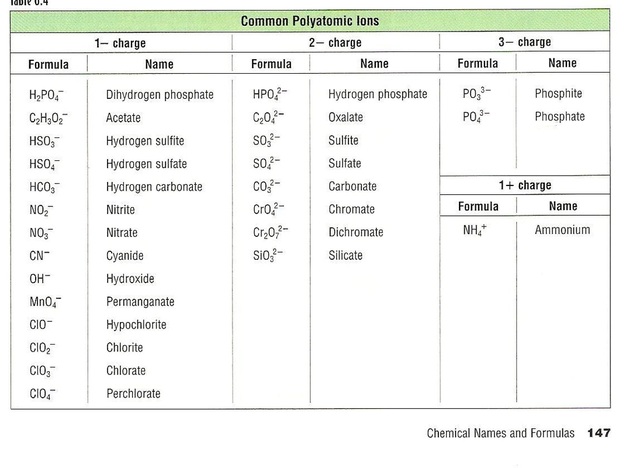
You are expected to know the names, formulas and charges of these ions. Notice that all but Ammonium are negatively charged.
Just as with the monoatomic ions, polyatomic ions form compounds by charge neutralization. The only difference is that when you need more than one polyatomic ion in a compound you must place parenthesis around it so that the reader knows the subscript refers to the number of entire ions, not just a single element.
For example:
Let's make Aluminum sulfate. Aluminum is a Group 13 ion so it forms +3 ions and Sulfate is a polyatomic ion SO42-. This means we need enough of each ion to make a neutral compound. The easiest way to figure this out is to determine the "least common denominator" for 2 and 3 or in other words what is the smallest number you can divide both 2 and 3 into evenly? By my calculations that number would be 6.
So Aluminums +3 goes into 6 two times and Sulfate's -2 goes into 6 three times. So the formula for a neutral molecule would be Al2(SO4)3. This means 2(+3) + 3(-2) = 0. Notice that the polyatomic ion is enclosed in parenthesis. This means that the subscript 3 refers to the entire polyatomic ion and that if you were asked how many of each element was in the entire compound you would have 3Al, 3S and 12O.
Ionic Nomenclature
Nomenclature is a fancy word that means "naming". The naming of ionic compounds for the most part is straight forward. You name the cation first by its elemental name and then you name the anion by adding the "-ide" suffix.
For example: A compound made of Magnesium and Bromine = MgBr2 = Magnesium Bromide
So when you combine any monoatomic ions, this is the process, BUT what about those polyatomic ions or those ions that have more than one possible charge? How are those handled?
Let's start with the polyatomic ions first:
When you look at the table of polyatomic ions you will see a pattern that forms between two ions of similar structure that only vary by the number of oxygens. The "-ite" ending means the form has less oxygens and the "-ate" ending means more oxygens.
Examples:
Nitrite = NO2- and Nitrate = NO3-
Sulfite = SO32- and Sulfate = SO42-
Compounds made with these polyatomic ions follow a similar format to the monoatomic ions but the suffixes do not change. NaNO3 is Sodium Nitrate and Na2SO3is Sodium Sulfite etc.
Another series that varies by the number of oxygens in the molecule are the Halogen and Oxygen compounds:
ClO- Hypochlorite
ClO2- Chlorite
ClO3- Chlorate
ClO4- Perchlorate
Because there are four versions of these ions there has to be more than just the previous 2 suffixes to separate them. This is done by adding the "Hypo-" and "Per-" prefixes. Hypo means the least number of oxygens in the series and Per indicates the most.
Finally we need to address the compounds we can make using elements that can form more than one ion. These are generally transition metal ions like Copper or Iron.
Whenever these ions are present in a compound you can determine their charge by the balance present.
Example: Cu2S or CuS, in the first compound since we know sulfur has a -2 charge as an ion, we can conclude that because there are two coppers present the copper must have a +1 charge. To indicate this charge in the name of the compound we use the roman numeral I in the name.
So Cu2S is Copper I Sulfide. In the CuS compound then the copper must have a +2 charge so the name would be written as Copper II Sulfide.
There is one other way you might see these compounds written that indicates the charge on the cation and that is by the old Latin names using "-ous" and "-ic" suffixes where "-ous" is the lesser charged cation.
So the compounds above would be written as Cuprous Sulfide for the Cu+ and Cupric Sulfide for the Cu2+
The best way to master nomenclature is to practice:
Chem21 Flash Cards on Naming Ionic Compounds
Back to HOME
Back to Segment 1