Molecular Formulas and Nomenclature
This is the content for Lecture 7 of General Chemistry. This lecture covers Covalent (Molecular) Compounds, Covalent Nomenclature and Acid formula and Nomenclature.
Molecular Compounds
Molecular compounds are formed when Non-Metal elements share electrons to form Covalent bonds. Because the bonds involve the sharing of electrons and not charge neutralization, there can be more than one structure created by the same elements:
Covalent Nomenclature
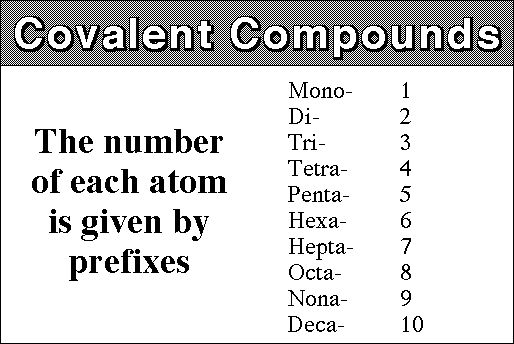
Covalent compounds simply use Greek numbers to state how many atoms of each element are present in the compound. You are probably already familiar with many of the Greek numbers since we use them in many ways already in our everyday language. E.g. The Pentagon, a Triangle or a Decade etc. Here are the numbers you will need to know from one to ten:
Here are some CHEM21 Flashcards on Molecular Nomenclature
Acids Nomenclature
Acids and bases are polar covalent molecules that dissociate into ions when introduced to water. According to the Arrhenius concept all substances which give H+ ions when dissolved in water are called acids while those which ionize in water to give OH-ions are called bases.
When naming acids there are several additional rules:
For simple binary acids containing only hydrogen and another element like HCl or HBr and with no oxygens present, you will use the prefix "Hydro-" to indicate the structure of the acid:

BUT when an oxygen is present you do not use the "Hydro-" prefix but rather change the suffix in such a way that "-ite" endings become "-ous" ending and "-ate" ending become "-ic" endings. See table above for some examples involving Sulfite and Sulfate.
Here are some CHEM21 Flashcards on Acid Nomenclature
Back to HOME
Back to Segment 1