
Geometric isomers are stereoisomers that are distinct and separate because they cannot freely rotate due to a multiple bond or a ring structure. Geometric isomers are generally not optical isomers unless they also happen to have chiral centers. These isomers are superimposable on their mirror images if no chiral centers are present. The isomers are diasteromers according to the definition given here.

There are two naming conventions. The older method uses cis- and trans- which works well for the example given above because there are two H atoms and two Cl atoms. But it won’t work well if there were four different atoms involved. The newer method ranks the substituents for each C atom according to the Cahn-Ingold-Prelog sequence rules. (Carey, Organic Chemistry pp. 268-272). If the two with the higher rankings are on the same side of the double bond, that isomer is Z (for the German word zusammen, which means together).
The other isomer is E (for entgegen meaning opposite). Briefly, the sequence rules rank the substituents in order of decreasing atomic number and if two or more atoms connected to the C atom are the same the second atom determines the order.
Provide systematic names for the following compounds, using the IUPAC rules as we discussed.
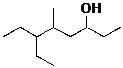

![]()
Define the geometry at the two double bonds shown by arrows in the molecule below. Make sure to write down the priority of each substituent around each double bond.


The R represents a carbon based group.