- If you flip an equation:
- If you add equations together:
- If you multiply an equation by a coefficient n:
Some more rules regarding the writing and manipulation of equilibrium constant equations:
Example: Calculate the K for the reaction of H and Br atoms to give HBr if given the following:
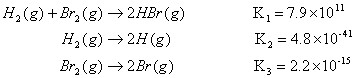
Answer:

| Converting Kc to Kp and Vice Versa: | |
|---|---|
•Kc –Equilibrium constant using concentrations •Kp –Equilibrium constant using partial pressures Kp = Kc (RT)Dn P = nRT/V R = 0.0821 L atm/mol K T = Temperature in K Dn = tot. mol product - tot. mol reactant |
Example 1: For 2SO3(g) <=> 2SO2(g) + O2(g) Kc = 4.08 x 10-3 at 1000 K. Calculate Kp. Kp = Kc (RT)Dn = 4.08 x 10-3 (0.0821 x 1000)1 Kp = 0.0335 --------------------------------------------------------------------------------- Example 2: For 3H2(g) + N2(g) 2NH3(g) Kc = 0.105 at 472°C. Calculate Kp. Kp = Kc (RT)Dn = 0.105 (0.0821 x 745)-2 Kp = 2.81 x 10-5 |
Disturbing a Chemical Equilibrium:
In 1888 Henri-Lewis Le Chatelier (1850 - 1936) a French industrial chemist made the observation: "Any change in one of the variables that determines the state of a system in equilibrium causes a shift in the position of equilibrium in a direction that tends to counteract the change in the variable under consideration." Simply put, Le Chatelier's Principle states that a system in equilibrium responds to any stress by restoring the equilibrium.
Types of Stress:
Example Calculation:
Changes in Temperature will change K
For an endothermic reaction increasing T increases K
For an exothermic reaction increasing T decreases K
For example, in the equation N2(g) + 3H2(g) <--> 2NH3 + 91.8 kJ, an increase in temperature will cause a shift to the left because the reverse reaction uses the excess heat. An increase in forward reaction would produce even more heat since the forward reaction is exothermic. Therefore the shift caused by a change in temperature depends upon whether the reaction is exothermic or endothermic.
Change in Pressure
When you increase the pressure, the system will shift so the least number of gas molecules are formed because the more gas molecules there are, the more collisions there are. These collisions and the presence of gas molecules are what cause the pressure to increase. Likewise, when you decrease the pressure, the system will shift so the highest number of gas molecules are produced.
For example, in the equation 2SO2(g) + O2(g) <--> 2SO3(g), an increase in pressure will cause the reaction to shift in the direction that reduces pressure, that is the side with the fewer number of gas molecules. Therefore an increase in pressure will cause a shift to the right, producing more product. (A decrease in volume is one way of increasing pressure.)
If you didn't look at this before, please do it now: