Intermolecular Forces
One of the biggest sources of difficulty for a chemistry student is the distinction between chemical bonds and intermolecular forces. While both are used to hold chemical systems together, they each introduce their own specific qualities into structures. This presentation is designed to draw basic comparisons between the two very different mechanisms.
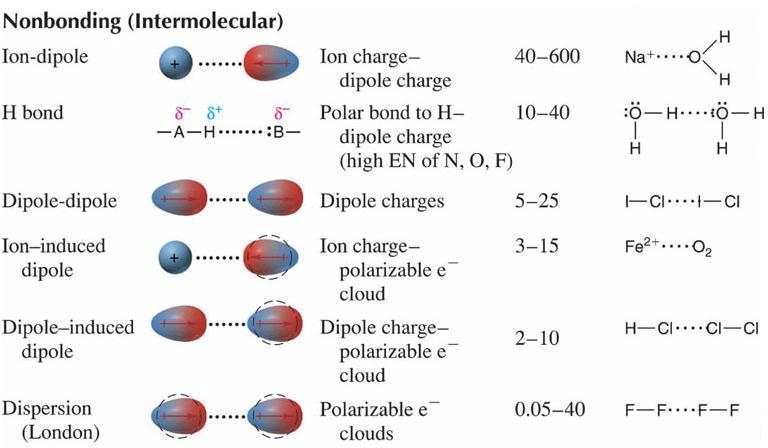
Bonds and intermolecular forces have one very fundamental thing in common. Both mechanisms are electrostatic forces of attraction (Coulombic forces) between areas of charge. The primary difference between bonds and intermolecular forces is the locations of the areas of charge and the magnitudes of the areas of charge. As a result of these differences, there are significant differences in the strengths of the resulting attractions.
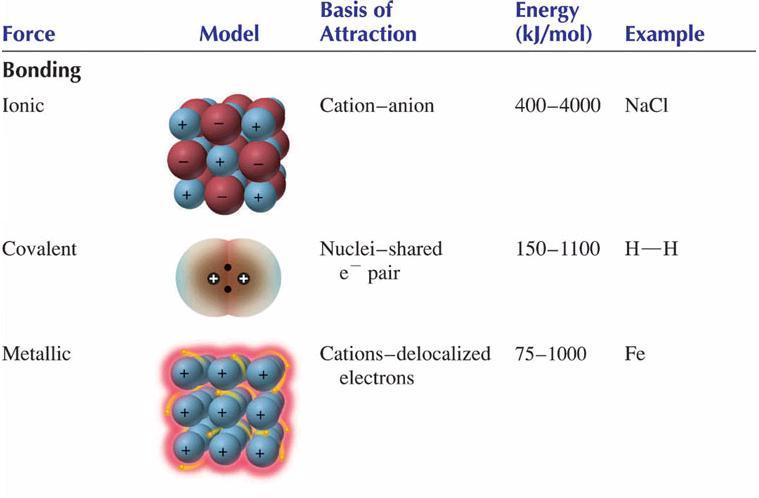
Chemists tend to consider three fundamental types of bonding:
Metallic bonding is a unique situation that is not as well understood as the more common forms of bonding, ionic and covalent. In this presentation, attention is focused primarily on ionic and covalent bonding.
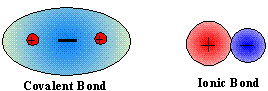
Because ionic and covalent bonding uses electrostatic attractions between areas of full charge, the resulting force of attraction is strong. Ionic bonds are held together by attractions between cations and anions. Covalent bonds are held together by attractions between positive nuclei and the negative electron clouds that reside between them.

The results of these bonding process are the strongest, commonly used, mechanisms for attaching atoms to one another. When a solid state chemical system is held together by bonds, ONLY, then the system will have qualities that are associated with this strong method of attachment. In other words, anything that only uses ionic or covalent bonding will have high melting points, high boiling points and be relatively hard and rigid.
There are two common examples of such systems. Quartz, or SiO2, is composed exclusively of covalent bonds. Table salt, or NaCl, is composed exclusively of ionic bonds.
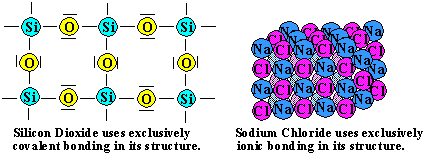
In both cases, the substances tend to be quite hard. In addition, they both exist as solids at room temperature because of their high melting points and boiling points. Some of the hardest substances known exist using bonding, exclusively, in their structures.
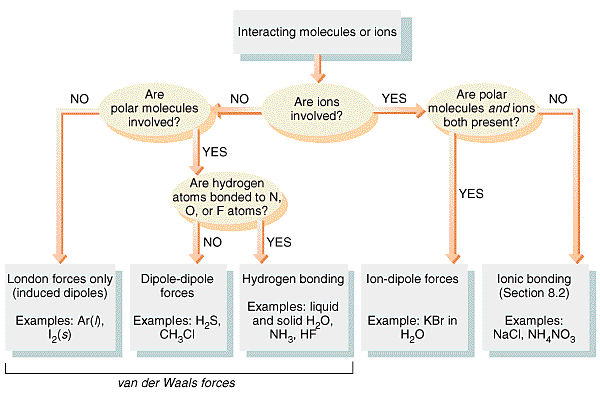
Intermolecular Forces:
These last two forces are collectively known as Van der Waals forces and are in general very weak.