

Let's revisit orbitals and basic atomic theory. What are they and how do they work with respect to bonding?
1) An orbital is a three dimensional description of the most likely location of an electron around an atom. Below is a diagram that shows the probability of finding an electron around the nucleus of a hydrogen atom. Notice that the 1s orbital has the highest probability. This is why the hydrogen atom has an electron configuration of 1s1 .


2) Orbitals are combined when bonds form between atoms in a molecule.
There are four types of orbitals that you should be familiar with s, p, d and f (sharp, principle, diffuse and fundamental). Within each shell of an atom there are some combinations of orbitals. In the n=1 shell you only find s orbitals, in the n=2 shell, you have s and p orbitals, in the n=3 shell, you have s, p and d orbitals and in the n=4 up shells you find all four types of orbitals. It is important to note here that these orbitals, shells etc. are all part of an empirical theory designed to explain what we observe with respect to molecular structure and bonding. As with any theory, these explanations will only stand as truth until someone (you maybe?) comes up with a better explanation or description.
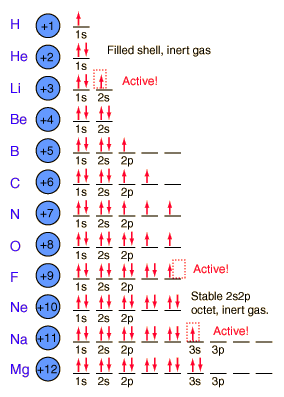
So here are a couple of pictures of atoms with their shells populated by electrons to help you remember this atomic theory. If you still need more review, the theory was presented in Kotz Chapter 7.

Orbitals and Electron Capacity of the First Four Principle Energy Levels |
||||
Principle energy level (n) |
Type of sublevel |
Number of orbitals per type |
Number of orbitals per level(n2) |
Maximum number of electrons (2n2) |
1 |
s |
1 |
1 |
2 |
2 |
s |
1 |
4 |
8 |
p |
3 |
|||
3 |
s |
1 |
9 |
18 |
p |
3 |
|||
d |
5 |
|||
4 |
s |
1 |
16 |
32 |
p |
3 |
|||
d |
5 |
|||
f |
7 |
|||
So now that we have reminded ourselves about the orbitals and how the electrons fill them, we need to address how these orbitals interact when two or more atoms bond together. For this process, we will discuss other theories: The Valence Bond Theory and The Molecular Orbital Theory.