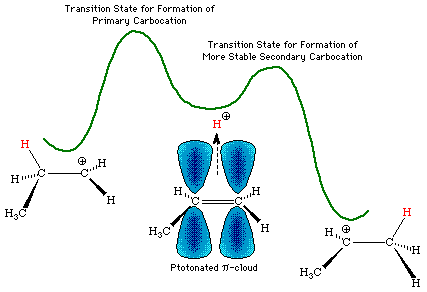
The addition of halogen acids to alkenes is a stepwise process which generally involves a solvent-equilibrated carbocation intermediate. The formation of this intermediate is initiated through a simple acid-base equilibrium in which the halogen acid donates a proton to the alkene p-system, which is functioning as a Lewis base. The protonated p-system has a short lifetime and can rapidly revert to starting materials, or can rearrange from a (cationic) protonated p-bond, to an sp3 sigma bond adjacent to an sp2 carbocation center. If the alkene is unsymmetrical, the protonated p-cloud intermediate can break down by two pathways, as shown below, to potentially form carbocations having differing ground-state energies. The reaction pathways leading from this intermediate to the two carbocations will differ in energy, and, in general, the pathway leading to the more stable intermediate will be of lower energy, and will be the preferred pathway.

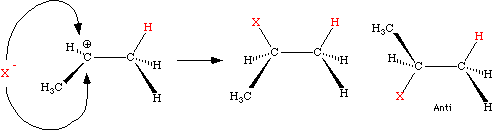
The resulting carbocation is formed on the carbon of the alkene which is best able to stabilize the cationic center. In simple unstrained non-conjugated systems, without adjacent heteroatoms, the order of stability of carbocations will be tertiary > secondary > primary. Since tertiary centers have no attached hydrogens, secondary centers have one and primary centers have two, there is an apparent inverse relationship between the "number of attached hydrogens" and the likelihood that the carbocation will form at that center. This is the origin of Markovnikov's Rule, which states that...
...in the addition of HX to an alkene, the proton will attach to the center having the greatest number of hydrogens...
often restated as "them that has, gets". While the rule is a useful guide, you should remember that the selectivity is actually to place the carbocation on the carbon that can best stabilize the charge.
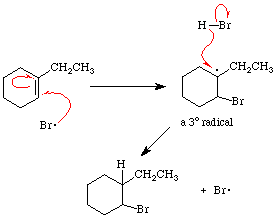
The addition of HBr to alkenes in the presence of peroxides converts the alkene into an alkyl bromide. The overall addition of HBr to the double bond is anti-Markovnikov, with the bromine being bonded to the alkene carbon which would form the least stable carbocationic center. The reaction involves attack of bromine radical to generate a radical on one of the alkene carbons. The selectivity arises due to the tendency to direct addition in order to form the most stable radical intermediate; rearrangements do not occur.
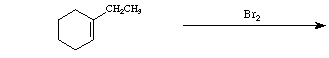
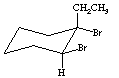
The addition of halogen to alkenes is a stepwise process involving a "halonium" ion intermediate. The formation of this intermediate is initiated through attack of halogen on the alkene p-system, to form the cyclic halonium ion (i.e., bromonium or chloronium ion) and expel the halogen anion (i.e., bromide or chloride). This intermediate is highly electrophilic and reacts rapidly with the best nucleophile in the system; that is, the halide anion expelled in the previous step. Since the halonium ion effectively blocks attack by halide on the same side, attack must be come from the backside (relative to the large halogen atom) to form the trans-1,2-dihalide.
Problems: Complete the following reactions