SAFETY NOTES: Acids and Bases can cause burns if they come in contact with your skin. Wash your hands immediately if you feel a burning or itching sensation. Use all necessary precautions when handling the acid and base containers.
GENERAL INSTRUCTIONS: This experiment is to be done in pairs, one partner will do the acid dilutions and the other the base dilutions. Be sure to get all of the information from your partner before leaving the lab
| Part I: Preparing Salt Solution | |
 |
|
 |
|
 |
|
 |
Place a watch glass over the mouth of the beaker if the salt starts to “spit” out. |
While the solution is boiling off, continue on to part 2. |
|
| Part II: Acid-Base Reactions and pH | |
 |
|
 |
|
 |
|
 |
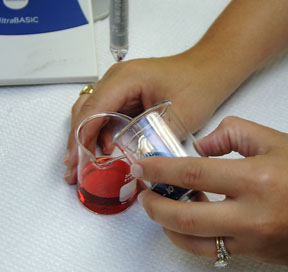
Using a graduated 1.0 mL pipette, the 25mL volumetric flask and either the acid or base, perform four serial dilutions using 0.5 mL of stock solution at each step. For example: Dilution one, pipette, using the three way bulb, 0.5 mL of 0.5M HCl OR 0.5 mL of 0.5M NaOH into the 25mL volumetric flask and dilute to the 25mL mark with DI water. Pour the solution into a clean beaker and then repeat until all dilutions are made. Calculate the resulting, NaCl, H+ and OH- concentrations as you did in your pre-lab. |
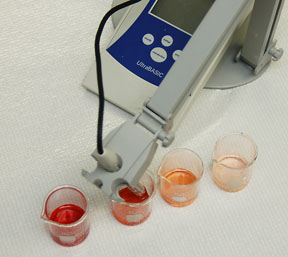 |
Record the pH of all 4 HCl dilutions and then of all 4 NaOH dilutions |
 |
|
| Part III: Experimental Yield | |
 |
|
 |
|
 |
|

