The Limiting and Excess Reagent Problem
As with any limiting and excess reagent problem we always must start with a balanced chemical reaction: 2H2 (g) + O2 (g) --> 2H2O (g)
This reaction is like a conversion table. It tells us that for every 2 moles of hydrogen we put into the reaction we will get 2 moles of water out or conversely if we got 10 moles of water as product then we must have used 5 moles of oxygen.. These statements are all based on the ratios of moles shown in the balanced reaction. If we know how many moles of a substance (reactant or product) we have, we can make predictions to answer any question the professor might throw at us.
The important thing to note about the above statements is that all of the predictions require one thing: THE AMOUNT OF SUBSTANCE MUST BE IN MOLE UNITS TO USE THE BALANCED EQUATION AS A CONVERSION TABLE.
So for the second part of our example problem we need to convert 100 grams of O2 and 100 grams of H2 into moles. This is done quite simply by dividing the gram amounts by the molecular weight of each element:
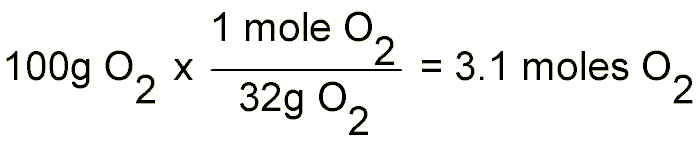
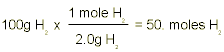
Now that we know how many moles of each reactant we have to work with, we simply use the balanced equation to answer any questions we might have:
If we put into the equation 50 moles of H2, this means that we will get 50 moles of H2O out. This is because the ratio between H2 and H2O is 2:2 or rather 1:1. If we put in 3.1 moles of O2, we will get 6.2 moles of H2O out, this is because the ratio between O2 and H2O is 1:2; i.e. for every one mole of O2 we put in we get 2 moles of H2O out.
Since 6.2 moles of water is much less than 50 moles of water, this means that we will run out of O2 much sooner than we will run out of H2 making O2 the limiting reagent. Whichever reactant can make the least amount of product is always the limiting reactant. This also means that 6.2 moles of water is the maximum amout of water we can make.
Another question that we can easily answer at this point is how much excess reagent, H2, is left over after the reaction. Well if we make 6.2 moles of H2O, this means we must have used 6.2 moles of H2 (remember that the 1:1 ratio goes both ways) and if we started with 50 moles and only used 6.2 of them, then 50 - 6.2 = 43.8 moles will be left unreacted.
If our question then asked us to report any of these values in grams, we simply reverse the process above and multiply by the molecular weight of each substance. I get that there will be ~112 grams of water and ~87.6 grams of H2 present at the end of the reaction. You try it and see if you don't get the same.