

CHM 1020--Chemistry for Liberal Studies--Fall 2000
 |
 |
|
CHM 1020--Chemistry for Liberal Studies--Fall 2000 |
|
Now
we turn our attention back to the electronic structure of atoms, and how this
electronic structure explains what holds atoms together in molecules, the attraction
we refer to as chemical bonds.
Let us recall two things we learned about
electron configurations from Chapter 3:
1.
The noble gases, which are essentially inert chemically, all have filled
s and p orbitals in the outer shell containing electrons.
In other words, they share the electron configuration s2p6.
2.
Families of main group elements have in common the number of electrons
in the s and p orbitals of the outer shell.
For example, all alkali metals have a single outer s electron.
Halogens all have an s2p5 configuration in the
outer shell.
From
these observations, we conclude that the important part of the electron configuration,
as far as chemistry is concerned, is the number of these outer shell electrons.
We refer to them as valence electrons.
A
rather simplified method of keeping track of these valence electrons, and of
getting some insight into how they are involved in chemical bonding, is the
use of electron dot structures, in which the symbol for each element is written
surrounded by dots for the valence electrons.
For
the main group elements, the number of valence electrons is given by the number
above the columns, and table 5.1 shows how these elements can be represented
by electron dot structures.
(It
is important to remember that electrons are not dots, and that our best picture
of electrons in atoms is a diffuse “cloud” showing the probable distribution
through space. This more complete
picture will be necessary to explain some aspects of chemical bonding, but the
simple “bookkeeping” of the electron dots can give us a lot of insight
into what factors are important).
Demonstrations:
Samples
of table salt and sugar are dissolved in water. The solutions are compared with pure water as to their ability
to conduct an electric current. (The
stronger the current, the more lights light up in the apparatus).
Pure water is compared with tap water.
Conclusion is that sodium chloride is an electrolyte, forming ions in
solution, while sugar does not. Tap
water contains some impurities that makes it a better conductor than pure distilled
water.
Alkali
metals Li, Na, and K are compared with their reaction with water.
The reactions are similar. Conductivity
shows ions are produced. An indicator
shows hydroxide ion (OH-) in each case.
Therefore they all have common chemical reactions.
They differ, though, in their reactivity.
The reactivity goes as follows:
K>Na>Li
We can express the reaction as follows:
potassium
+ water ®
potassium ion + hydroxide ion + hydrogen gas.
Using chemical symbolism, this becomes:
K + H2O ® K+1 + OH-1 + H2
But to be a proper chemical reaction, it must be balanced. The same number and kind of atoms must appear on each side of the equation.
Note there are too many H’s on the right. We can correct that by putting a 2 in front of H2O.
K + 2 H2O ® K+1 + OH-1 + H2
But this now puts too many H’s on the left, as well as too many O’s. We can correct that by putting a 2 in front of OH-1.
K + 2 H2O ® K+1 + 2 OH-1 + H2
Now all the atoms are balanced, but there is another problem. The charges must be balanced as well. There are two negative charges on the right, but only one positive charge. So we can correct this imbalance by putting a 2 in front of each K:
2 K + 2 H2O ® 2 K+1 + 2 OH-1 + H2
We will do more balancing in the next chapter.
This
is a common type of correlation among families in the periodic table.
All elements in the same family tend to undergo the same reaction, including
the same relative numbers of atoms in a compound, but there may be a trend in
reactivity from one element to another.
So
how are we going to invoke our electron dot structures to begin to explain these
reactions?
First,
we classify compounds into two broad general categories (with some overlap):
· ionic compounds (like sodium chloride. Contains ions and aqueous solutions conduct an electric current).
·
covalent compounds
(like table sugar. Solutions
do not conduct an electric current).
Ionic
Compounds---formation of ionic bonds.
Metals
tend to lose electrons to form positive ions.
This can be best understood with the metals to the extreme left of the
periodic chart—the alkali metals and the alkaline earth metals.
By losing their valence electrons, they obtain an electron configuration
like that of the previous noble gas:
![]()
![]()
When
non-metals form ions, they tend to gain electrons to form negative ions. This is best illustrated by the halogens, which can gain one electron
and obtain a rare gas configuration. Oxygen can do so by gaining
two electrons.

![]()
Transition
metals are a bit more complicated since many of them can lose different numbers
of electrons to form positive ions with different sized charges.
(Fe+2 and Fe+3
for example).
Some
of the common ions are illustrated in Figure 5.4.
The
formula for ionic compounds is simply dictated by the charges on the ions.
The number of positive and negative charges must balance.
So:
Ionic
compounds do not contain distinct molecules. Instead they consist of a lattice of alternating positive and
negative ions. In sodium chloride
for example, each sodium ion is surrounded by six chloride ion, and each chloride
ion is surrounded by six sodium ions.
Ionic compounds tend to be very high melting, reflecting the great amount
of energy necessary to separate the ions from each other.
The
formula for ionic compounds is referred to as an empirical formula.
It gives the relative number and kinds of atoms in the compound.
Naming Ionic Compounds
Ionic compounds are named by simply naming the ions that make up the compound. So you need to learn the names of the ions. Most are pretty simple. Refer to Table 5.2 for the names of common monatomic ions.
Cations (positive ions) are simply given the name of the element. For alkali metal, alkaline earth metal, and aluminum, that is all you need to use, because they form only one kind of ion. We pointed out that the transition metals can sometimes form more than one ion, and in that case you need to designate which one you are talking about. There are two ways of doing the, the modern Stock nomenclature, which simply puts the charge in parenthesis after the ion, but in Roman numerals. An older method uses the suffixes ous and ic to differentiate between the lower and higher charge when two are possible. So, for example:
Ion
|
Stock
name |
Old
name |
|
Cu2+ |
copper (II) |
cupric |
|
Cu+ |
copper (I) |
cuprous |
|
Fe2+ |
iron (II) |
ferrous |
|
Fe3+ |
iron (III) |
ferric |
Note that you just have to learn which charge ion each forms, and which are the ous and ic forms. Note also that the ous and ic nomenclature is used with the Latin names of the elements.
Anions (negative ions) add the ending ide to the stem of the element name. Chlorine becomes chloride, bromine becomes bromide, etc. Sometimes the stem is changed slightly—oxygen becomes oxide.
Covalent
compounds on the other hand achieve electronic stability by sharing rather than
gaining or losing electrons.
Take
the diatomic element hydrogen, for example.
When a bond is formed between two hydrogen atoms, both atoms have the
same tendency to gain or lose an electron, so instead of one giving up to the
other, each obtains the configuration of helium by sharing electrons.
A
covalent chemical bond is illustrated by showing a shared pair of electrons
between two elements. Showing the
shared pair is equivalent to drawing a single bond connecting the two elements:
![]()
So
the Cl2 molecule would be represented as:

The tendency to share, rather than gain
or lose electrons, is not a black and white, all or none, phenomenon. There are shades of gray. At the extremes are the purely ionic compounds, where the non-metallic
element (Cl for example) has such a stronger attraction for electrons than sodium
that it takes the electron completely away.
At the other end are the cases of bonds between identical elements. neither
wins out over the other, so the electrons are shared equally.
The shades of gray come into play when one
element has a stronger tendency to gain an electron than the other, but not
strong enough to completely strip the electron away.
In that case a polar covalent bond is formed.
Such is the case with HCl. Cl has a stronger attraction for electrons than H, so that
the electrons shared between them are most often associated with the chlorine.
The molecule acts as a little magnet, with a partial positive charge
on one end (the hydrogen end), and a partial negative charge on the other.
Figure 5.6 shows an illustration of the unequal distribution of electrons
about the HCl molecule.
Naming Covalent Compounds
Many of these compounds have common names. We have already discussed water (H2O), ammonia (NH3) and methane (CH4).
Binary inorganic compounds (consisting of just two elements) are named using Greek prefixes to indicate the number of a particular atom in the molecule. Prefixes are given in table 6.3. (mono=1, di=2, tri=3, tetra=4, penta=5, hexa=6, hepta=7, octa=8, nona=9, deca=10). We often omit the mono for the first element in the name.The name of the element farthest to the left in the periodic table is usually written first. If both are in the same group, the lower one is named first.
The name of the second element is given the ide ending.
Take a look at problems 82 and 83 on page 147.
Covalent organic compounds (compounds of
carbon) are much more complicated, and require not only a chapter but a course
all their own. (Methane is one
example). We will cover some of
these later.
The relative tendencies to attract electrons
in a covalent bond are measured by a property called electronegativity.
Electronegativity
This tendency to gain or lose electrons can be summarized in a property of the atoms known as electronegativity. This property is a complex combination of nuclear charge (the greater the nuclear charge, the greater the attraction for an electron), and distance from the nucleus (the further the electron orbital is on average from the nucleus, the less the attraction).
Electronegativity increases as you progress across a period. (The average distance remains the same, the nuclear charge increases).
Electronegativity decreases as you go down a family. (While the nuclear charge increases, the electrons are found in higher shells, further from the nucleus).
Formation of ions represents an extreme case. More often, we see cases where atoms share electrons, and depending on electronegativity, the sharing may be equal or unequal. When electrons are shared by two atoms, we say that a covalent bond is formed. The shared electrons act like glue, being attracted to the nuclei of both atoms, tending to hold them together.
Sharing of electrons is most common between non-metals, or when hydrogen is combined with a non-metal.
Electrons are shared equally between identical atoms (H2, Cl2, etc_)
When electrons are unequally shared, the produce a polar bond. In the case of a diatomic molecule, this is a molecule like a little magnet, with a partial positive charge on one end, and a negative charge on the other. Examine the representation of HCl in Figure 5.6.
Sharing of electrons among more than two
atoms, to form polyatomic molecules is only slightly more complicated. But most of the possible structures can be predicted with a
few simple rules for writing Lewis structures.
Lewis dot structures for polyatomic molecules.
The rules for sharing electrons are simple. Each atom in the compound combines in such a way as to achieve a stable octet (i.e. like the s2p6 configuration of a noble gas) by sharing electrons. This relationship was first recognized by G.N. Lewis, and the structures of covalent compounds drawn in such a way as to illustrate this point are called Lewis dot structures.
First lets take a look at the period 2 elements C, N, O, and F with H.
C has only 4 valence electrons. It requires four more to make 8, and can get those from hydrogen by sharing only if it combines with four hydrogen atoms. Hence the stable compound formed between C and H is CH4.
Nitrogen has 5 valence electrons. It can obtain eight by sharing with three hydrogens. Hence NH3.
Water has 6 valence electrons. It can obtain eight by sharing with two hydrogens. Hence H2O.
F needs share only one, so we get HF.
From this we can draw some simplifying conclusions:
These compounds exist in discrete molecules, and the formulas represent the actual number and kind of atoms in the molecule. Hence these formulas are called molecular formulas, as distinct from the empirical formulas of ionic compounds.
(Aside: Hydrogen peroxide exists as a molecule with the molecular formula H2O2. That would be its molecular formula. Its empirical formula would simply be HO).
Although the model is not perfect, we can use Lewis dot structures to predict the composition and structure of most covalent compounds.
Rules for writing Lewis
structures:
Go through examples:
Second row elements with hydrogen:

Carbon dioxide, 16 valence electrons (4+6+6)
![]()
Sulfur dioxide, 18 valence electrons (6+6+6)
![]()
Note in this case, two different distributions of electrons are possible. We call these resonance structures, and the "real" structure is sort of a mixture of the two.
Go through more examples:
H2, Cl2, H2O, NH3, CH4, CO2, CO, SO2, SO3, NO, O2, O3
(See Figure below for drawn examples)
Note structures where two or more electron distributions can be written are called Resonance Structures. The actual structure is sort of a hybrid of these as extremes.
Note also that the model is not perfect. There are some cases where the stable octet rule is not obeyed:

Polyatomic ions
Many compounds contain both ionic and covalent bonds. Collections of atoms held together with covalent bonds, yet carrying a net charge, are called polyatomic ions.
Polyatomic cations are very few. You need concern yourself only with ammonium (NH4+) and hydronium (H3O+).

There are three polyatomic anions with common names that you should learn: hydroxide (OH-), cyanide (CN-) and acetate (CH3CO2-).

Note you can draw Lewis dot structures for these ions just like for covalent compounds. If the ion is positive, you need to deduct one or more electrons in calculating the number of valence electrons in the structure. If the ion is negative, you need to add one or more electrons to the number for the structure.
The rest of the polyatomic anions that concern us are oxyanions, that is they contain one or more oxygens surrounding a central non-metal atom. These ions are named by adding the ending ate to the stem for the non-metal atom.
SO42-
sulfate
ClO3-
chlorate
PO43-
phosphate
NO3-
nitrate
It is also easy to draw Lewis structures for these oxyanions.


There is no standard pattern here relating number of oxygens or charge. You just need to learn the names of these principle oxy-anions.
Once you have learned these names, though, you can relate the names of several others.
If there is one less oxygen than normal, the ending is ite.
(Give the structures of:
Many of these polyatomic ions are given in Table 5.4. Learn these names as well. You should be able to draw Lewis structures for these ions, and predict their geometry, just as we did for the neutral molecules in the previous chapter.
Note some have an additional hydrogen. HCO3-1 is called hydrogen carbonate (or bicarbonate, where the prefix "bi" refers to the hydrogen atom).
Molecular Geometry
Structural formulas only tell us the order in which atoms are connected in a structure. A geometrical formula is required to get a sense of the 3-dimensional distribution of the atoms in space. Fortunately, there is a very simple model, equivalent to the Lewis dot structure model for predicting structural formulas, that can give us insight into this question. It is called the valence shell electron pair repulsion (or VSEPR) model.
The VSEPR model simply states that pairs of valence electrons around a central atom tend to distribute themselves in space in a way to get furthest away from each other. For example:

This is referred to as the Electron Pair Geometry.
The Molecular Geometry will depend on whether all pairs are bonded to an atom, or whether there are lone pairs not bonded to an atom. Lets compare the three compounds methane (CH4), ammonia (NH3) and water (H2O). All have four electron pairs around the central atom, but varying numbers of lone pairs. Note that in the molecular geometry, the electron pairs do not show up.
Compound |
No. of Electron Pairs |
Geometry |
Structure |
||
|
Bonding |
Non-bonding |
Electron pair |
Molecular |
||
|
CH4 |
4 |
0 |
tetrahedral |
tetrahedral |
|
|
NH3 |
3 |
1 |
tetrahedral |
trigonal pyramid |
|
|
H2O |
2 |
2 |
tetrahedral |
bent |
|
The Chime plug-in for your web browser gives you the ability to rotate and otherwise manipulate 3-D molecular structures. For some practice on these simple molecular structures, check the molecular geometry link on the course page. Practice trying to represent these 3-D structures in a 2-D drawing.
Shapes and Properties
Why are shapes important? When knowledge of the shape is combined with knowledge of the polarity of the bonds in the molecule, we can conclude whether the whole molecule is polar or non-polar. The degree of polarity of the molecule as a whole affects intermolecular forces, which describe how strongly molecules are attracted to each other, therefore determining how much energy must be put in to pull them apart.
For diatomic molecules, the reasoning is simple. If there is a sufficient difference in electronegativity between the two atoms, the bond is polar, and the molecule as a whole is polar. It is often represented as a small arrow pointing in the direction of the negative charge (toward the negative end of the molecule):
![]()
For polyatomic molecules, the issue is more complicated because the small dipole arrows add together as vectors, which show both a magnitude (represented by the length of the arrow) and direction. To add vectors, just lay them end to end, and the resultant vector sum is the distance and direction from the start of the first arrow to the end of the last one. For example:

Sometimes the vector sums will be zero:

So for unsymmetrical molecules, if the bonds are polar, there will be a net dipole and the molecule will be polar:

If the molecules are symmetrical, even though the bonds are polar, the molecule will be non-polar:


Some molecules may be non polar, even if they are unsymmetrical:

With this discussion as background, let's now turn to consideration of how these properties of molecules help us understand about how molecules interact with each other. First recall our earlier discussion (Lecture 2) on states of matter. At the time, we suggested a model to explain the different behavior of the three states, and this model is known as the kinetic-molecular theory.
From this kinetic-molecular theory for gases, we extrapolate to liquids and solids. In solids, the particles are in highly ordered assemblies in close contact with one another, and unable to move relative to one another. In liquids, the particles remain in close contact, but are much more loosely organized and are free to move relative to one another.
It requires energy to pull molecules of a solid apart enough so that they are free to move as a liquid. It takes even more energy to pull them much further apart in to form a gas. This energy is used to overcome the attraction between molecules, an attraction that is the result of what we call intermolecular forces.
Lets illustrate this energy input by examining the heating curve for water. This curve is very much like the more generalized curve in Figure 5.12 of your book.
Beginning with ice at –40 oC, as you add heat energy, the temperature of the ice increases until it reaches 0 oC, the melting point of ice. The increasing temperature is reflecting energy going into the vibrational and rotational energy of the molecules, i.e. the kinetic energy of the molecules. The temperature response of ice to heat is measured as the specific heat, which for ice is 0.50 calories/gram-oC or 2.1 Joules/gram-oC.

Then the temperature remains constant as more energy is put in. This corresponds to an increase in potential energy of the molecules, as they are pulled apart slightly against an attractive force. The amount of energy required here is 80 calories/gram, which is called the heat of fusion. Liquid water then increases in temperature as more energy is put in, with a specific heat of 1.0 calories/gram-oC. (Note, this is, in fact, how the calorie was first defined). When the temperature reaches 100oC, it takes a great deal more energy to pull the water molecules completely apart. This is known as the heat of vaporization, which is 540 calories/gram.
The graph is reversible, which means as steam is cooled, heat is given off. The very large heat of vaporization means that when steam condenses, it lets out a large amount of heat, which is why one can get burned so easily with steam. (Just the opposite of the cooling effect when liquid water evaporates from the skin).
Different substances, of course, will have different melting and boiling points, and different heats of fusion and vaporization. These will depend on the strength of the forces holding the molecules together in the solid and liquid state.
There are several kinds of forces acting between particles, and they vary in their strength. We will consider the following:
Ionic Forces
Ionic forces are the strongest. The force of attraction depends on the size of the charge on the ion and the distance between them. These are called coulombic forces. Ionic compounds, such as sodium chloride, are high melting (812 oC), and very high temperatures are necessary to vaporize the ions (1413 oC). Recall that in the solid, each ion is surrounded by several ions of opposite charge.
Ion-Dipole Forces
These are the next strongest forces. You will only have these interactions in solutions, most commonly solutions of ionic substances in water. The interactions of the dipole of water with the ions must be strong enough to help break apart the lattice arrangement of the ions in solution.

We refer to this interaction as the solvation of ions by the solvent water. Notice each positive ion is surrounded by the negative end of the polar molecule, and each negative ion is surrounded by the positive end of the polar molecule.
Dipole-Dipole Forces
Polar molecules can behave like little magnets, with the positive end of one molecule attracted to the negative end of the other. A variety of such arrangements are possible:

Hydrogen Bonding
When hydrogen atoms are attached to very electronegative, very small atoms (specifically F, O, and N), the polarity of the bond is very great, and the positive end of the bond at the hydrogen atom is capable of getting very close to and interacting with the lone pairs on other F, O, or N atoms.
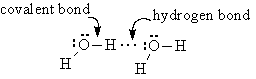
These bonds are considered special because the strength of attraction is quite a bit more than the attraction between dipoles. Lets look at the trend in boiling points for the hydrogen compounds of the halogens and the oxygen family:
|
Compound |
B.
P. (oC) |
Compound |
B.
P. (oC) |
|
HF |
19.5 |
H2O |
100.0 |
|
HCl |
-84.9 |
H2S |
-60.7 |
|
HBr |
-67.0 |
H2Se |
-41.5 |
|
HI |
-35.4 |
H2Te |
-2.0 |
Notice that the trend is increasing boiling point as the molecular weight of the compound gets larger, except for HF and H2O. These both have unusually high boiling points, attributed to the hydrogen bonding that can occur between these molecules. (Remember, only hydrogens attached to F, O, or N can participate in hydrogen bonding).
In ice (solid water), the hydrogen bonding between molecules forms a tetrahedral bonding structure. This bonding gives ice a very open structure, as illustrated by this chime figure. (Illustrate ice.pdb file in class). Melting of ice breaks some of the multiple hydrogen bonds, allowing the molecules to move relative to one another, but still many of the bonds remain. However, as they can move, they can on average get closer together, so that on melting, the liquid water becomes more dense than the solid. Hence ice floats. (If it didn't, the conditions for developing life on earth might be quite different).
So the hydrogen bonding of water explains these unusual properties of water:
Dispersion Forces
What holds non-polar molecules together? Even very non polar atoms like helium can be liquefied at a low enough temperature. The explanation for these forces is a bit more complicated.
Let's first look at helium atoms (2 electrons). Consider the particle nature of electrons

These are referred to as London dispersion forces They are much weaker than the other forces, but they increase as the size of the atom increases, and as the total number of electrons in the atom or molecule increase. Therefore dispersion forces get stronger as molecular weight increases, and they are what is responsible for boiling points increasing with molecular weight. (Refer back to the comparison of HCl, HBr, and HI, which decrease in polarity as you go to higher molecular weight, but increase in boiling point).
Solutions
Whether substances can mix to form solutions depends on these properties. In general, substances mix best when they have similar bonding properties. (Like dissolves like). Consider gasoline, which consists of a mixture of hydrocarbons such as octane:

The C-H bonds are not very polar, and the tetrahedral arrangement tends to cancel out what little polarity exists, so hydrocarbons are not polar. They are held together by dispersion forces. Hydrocarbons will dissolve other non-polar substances, such as grease, but will not mix with water. Whereas water can solvate polar molecules and ions, they interact with themselves more strongly than they can interact with a non-polar molecule.
Molecules with OH bonds, like ethyl alcohol and the sugar glucose, are not only polar, but they also can participate in hydrogen bonding with water, so they are soluble in water.

|