

CHM 1020--Chemistry for Liberal Studies--Fall 2000
 |
 |
|
CHM 1020--Chemistry for Liberal Studies--Fall 2000 |
|
Balancing Chemical Equations
A chemical change is described by writing a chemical equation.
reactants ® products
The reactants are the substances present before the reaction, and the products are the substances that are formed in the reaction.
Both reactants and products are represented by their chemical formulas. Empirical formulas are used for ionic compounds, while molecular formulas are used for covalent compounds. (One type of chemical equation called the net ionic equation is sometimes used for reactions of ions in aqueous solution, where the formula for the ions is given rather than the complete compound. We won't worry about that type just yet.)
These equations provide us with quantitative information about the reacting substances. Recall from the Law of conservation of mass that the mass of the products must equal the mass of the reactants, i.e. there is no change in total mass in a chemical reaction. For the equation to properly express this relationship, the equation must be balanced. That is there must be the same number and kind of atoms on each side of the arrow. And of course, the formulas themselves must be correct.
Lets look at a few examples:
First the one from a demonstration we did a few lectures ago:
Li + H2O ® LiOH + H2
It is sometimes common to indicate the state of the substance (s for solid, g for gas, l for liquid, aq for aqueous solution) but your textbook doesn't bother to make those distinctions, so we won't either.
The usual approach is sort of trial and error, which works fine unless the equation is very complicated. Go through one atom at a time, making changes to get the correct number on both sides. Sometimes you have to backtrack. The important thing is that you can only change coefficients of substances. You cannot change subscripts, because that would change the nature of the substance. Following might be a train of thought:
Li balanced, O balanced, H not balanced. Need more H on the left, so put 2 in front of H2O. Now both H and O are unbalanced, but can correct by putting a 2 in front of LiOH. Now Li is unbalanced, so put a 2 in front of Li on left. Finally you get:
2 Li + 2H2O ® 2 LiOH + H2
Lets try a few: (The final coefficients are indicated in the parentheses to the right of the equation).
This latter reaction is called a combustion reaction, and it represents a type of reaction where you can usually easily predict the products. Complete combustion of any compound containing only C, H, and O, will produce CO2 and H2O as products.
So see how you can do with the following combustion reactions:
Now lets do at your seat some of the ones at the end of the chapter: # 25 page 180. Then practice on your own for some others, and if you still want more practice, here is a web site you can go to.
Volume Relationships
One of the early quantitative relationships recognized in chemical reactions had to do with reactions of gases. Gay-Lussac carried out a number of measurements with reacting gases, and he noticed a relationship that he summarized in his Law of combining volumes: Volumes of gaseous reactants and products, if measured at the same temperature and pressure (important!), are in the ratio of small whole numbers.
Whenever there is a simplifying law like this, one can usually suspect some fundamental underlying cause. The underlying cause was explained by the Italian chemist, Amadeo Avogadro, and it is called Avogadro’s Hypothesis. It is a very simple hypothesis: Equal volumes of gases at the same temperature and pressure contain the same number of molecules.
This should almost be intuitive, and is explained in terms of the balanced chemical equation. For example:
2 H2 + O2 ® 2 H2O
The gases hydrogen and oxygen react in the volume ratio of 2:1, and if the reaction were carried out at a temperature at which water was a gas, then 2 volumes of water would be formed. The balanced equation tells us that 2 molecules of hydrogen react with one molecule of oxygen. So let’s say one volume (one liter, for example) of oxygen contained x particles, then according to Avogadro’s hypothesis, two liters of hydrogen would contain 2x particles, giving the reaction ratio of 2x H2 reacting with x O2. Note Figure 6.3 and Figure 6.4 showing these relationships visually.
The same reasoning would apply to the reaction of nitrogen and hydrogen to form ammonia:
N2 + 3 H2 ® 2 NH3
Note from Figure 6.3 that one volume of nitrogen reacts with three volumes of hydrogen to give 2 volumes of ammonia, again the same whole number ratio as the coefficients in the equation.
This reasoning, by the way, was what helped establish the understanding that some elements like hydrogen and oxygen were diatomic, a fact that Dalton never accepted. How does it do that? Suppose they were monatomic, and the equations were:
Then if Avogadro’s hypothesis were correct, 2 volumes of hydrogen would still be reacting with one volume of oxygen, but should produce one volume of gaseous water, not two. And 3 volumes of hydrogen would react with one volume of nitrogen, but would produce one volume of ammonia, not two.
More about gases in a moment. Let’s next consider the weight relationships in a chemical reaction.
We can calculate the relative weights of molecules using the atomic weight scale, and can do so without having to know the absolute weight of an atom. On the atomic weight scale, H2 would weigh 2 amu (atomic mass unit), O2 would weigh 32 amu, and H2O would weigh 18 amu.
With ionic compounds, like NaCl, in which individual molecules don’t exist, we can still calculate the relative weight of the formula unit NaCl, called the formula weight, as 23.0 + 35.5 = 58.5 amu.
Note that your textbook uses the terms atomic mass, molecular mass, and formula mass to mean the same thing, and u to stand for the atomic mass unit. I will probably use the terms interchangeably--more likely using atomic and molecular weight.
Most of you have had chemistry and I’m sure have done such exercises before, but let’s do a few at your desk to make sure you understand the principle. Calculate the molecular weight (MW)or formula weight (FW) of the following (and indicate which it is).
(Note that atomic weights of many elements are known to several significant figures, and in very accurate work, one should use the most precise values available. For simplicity, it is sufficient for our exercises to round to 0.1 amu to carry out calculations.)
So now lets go back to our simple reaction between hydrogen and oxygen to look at relative weights of reacting substances:
2 H2 + O2 ® 2 H2O
2*2.0 amu + 32.0 amu 2*18.0 amu
4.0 amu 32.0 amu 36.0 amu
Note there are 4.0 + 32.0 amu on the left, 36.0 amu on the right, obeying the law of conservation of mass. So the relative weight ratios are 4:32:36.
If that’s the case, how much oxygen would be required to react with 4 pounds of hydrogen? (ans. 32 pounds) What about 4 grams of hydrogen? (ans. 32 grams). What about 2 grams of hydrogen? (ans. 16 grams).
Relationships like this can be solved several ways, one of the simplest being ratio and proportion. A proportion is an equation in which two ratios are set equal.
Restating the question, “How many (?) grams of oxygen would be formed from 2 grams of hydrogen if 32 grams of oxygen are formed from 4 grams of hydrogen?” gives the following proportion:
![]()
Remember, though, that the ratio of reacting particles was 2:1:2. So in a reaction which involves N particles of O2 molecules
We can begin to see a pattern here. 2 g of H2, 32 g of O2 and 18 g of H2O all have something in common: they all contain N molecules (or particles).
Therefore chemists have developed a new measure of amount of substance which measures the amount in terms of numbers of particles, rather than in terms of mass.
This amount of substance was first defined as the gram molecular weight, which was the weight, taken in grams, equal to the molecular weight (in amu) of the substance. Likewise a gram formula weight would be a weight of an ionic compound, taken in grams, equal to the formula weight, and a gram atomic weight would be the amount of an element, taken in grams, equal to the atomic weight of the element. These quantities all have in common that they contain the same number of particles. How many particles? At first the number was unknown, and simply given the value N, which was called Avogadro’s Number.
For simplicity, the terms gram molecular weight, gram formula weight, and gram atomic weight have all been simplified to one term, the mole. A mole of a substance contains N particles of that substance.
Note some subtleties here. A mole of hydrogen gas (H2) weighs 2 g, and contains N molecules of H2. A mole of hydrogen atoms (H) weighs only one gram, and contains N atoms of H. So when dealing with diatomic elements, it is necessary to specify whether one is talking about a mole of molecules or a mole of atoms.
For ionic compounds like NaCl, a mole of NaCl, which is 58.5 g, contains a mole (N) Na ions and a mole (N) Cl ions, or a mole (N) of “formula units”.
For quantitative calculations of chemical reactions, it is not necessary to know the size of N. But since the first development of this concept, we have been able to measure a value for N, actually to as many as eight significant figures, as the value 6.0221367 x 1023. For our purposes, it is usually sufficient to use the value 6.02 x 1023. Like all measurements, this number has a unit: 6.02 x 1023 particles/mole, where “particles” can mean atoms, molecules, formula units (or even in exercises to try to grasp the magnitude of the quantity—grains of sand as in a “mole of sand grains”).
Remember! The mole is amount measuring a number, just as dozen and gross measure numbers of things. A dozen eggs will have a different mass than a dozen grapefruit, but they both contain 12 items. A mole of carbon has a different mass as a mole of sulfur—but they both contain the same number (6.02 x 1023) of atoms.
As measurements became more precise, the definition of the mole had to become more precise, It is now defined, in the SI system of measurement, as the quantity of substance that contains the same number of particles as there are carbon atoms in exactly 12 grams of isotopically pure carbon-12. (Remember, the current reference point for the relative atomic weight scale assigns carbon-12 a mass of exactly 12 amu, with everything else measured relative to that number). Putting this in other words, Avogadro’s Number is the number of carbon atoms in exactly 12 grams of isotopically pure carbon-12.
This relationship gives us a second unit to use in talking about atomic, molecular, and formula weights. Instead of saying the molecular weight of water is 18.0 amu, we can also say it is 18.0 grams/mole. In other words, the molecular weight (or formula weight, or atomic weight) is also the molar mass.
Let’s practice a few conversions of mole to mass, and mass to mole. Try problems 44 and 45, page 181.
You can also get some practice in such conversions at George Wiger’s interactive web site.
Molar Volume
Now some of you may be astute enough to link this concept back to what we had to say about reacting volumes of gases, explained by Avogadro’s hypothesis. Recall that Avogadro suggested that equal volumes of gases, measured at the same temperature and pressure, contain the same number of particles. A corollary of this is that quantities of gas containing the same number of particles should occupy the same volume. In other words, a mole of one gas, containing N particles, should occupy the same volume as a mole of another gas, also containing N particles. Therefore at any given temperature and pressure, there should be a characteristic molar volume, and there is. At standard temperature and pressure (STP), which is 1 atmosphere pressure and 0oC, the molar volume is 22.4 L.
This makes it easy to calculate molecular weights of gases by measuring the density of a gas. Suppose you measure the density of a gas to be 2.86 g/L at STP. What is its molecular weight?
2.86 g/L * 22.4 L/mol = 64.1 g/mol
What is the density of neon gas at STP?
20.2 g/mol * 1 mol/22.4 L = 0.90 g/L
Do a few more for practice: (35-40, page 181).
Mole and Mass Relationships in Chemical Reactions
Next let us see how we can use balanced chemical equations to calculate the relative amounts of substances that undergo a chemical reaction. The coefficients of a chemical reaction specify the relative number of molecules (or formula units) that react. They also represent the relative number of moles that react. From the molecular or formula weights, we can determine the relative number of grams that react.
So lets look at number 7 on page 181.
The first thing one must do is write the proper balanced equation. In this case we are talking about combustion of octane. From the balanced equation, one can determine the relative number of moles reacting, giving in essence a conversion factor or chemical equivalency between each component of the reaction.
2 C8H18 + 25 O2 ® 16 CO2 + 18 H2
2 moles 25 moles 16 moles 18 moles
How many moles CO2 are produced when 2.09 mol of octane is burned?
![]()
Here the ratio 16 mol CO2/2 mol octane is the chemical equivalency used as the conversion factor.
For part b:
![]()
To do a calculation involving a mass of reactant or product, you must first convert the mass to moles, convert by the chemical equivalency, then convert back to grams.
For example, number 51, page 182, which asks about converting quicklime to limestone. How much quicklime (CaO) can be made from 4.72 x 109 g of limestone (CaCO3). First we need to calculate the formula weights of these substances.
FW CaO = 40.1 + 16.0 = 56.1 g/mol
FW CaCO3 = 40.1 + 12.0 + 3 x 16.0 = 100.1 g/mol
Then we write the balanced equation:
CaCO3 ® CaO + CO2
and solve the problem by the following series of conversions:
g CaCO3®mol CaCO3®mol CaO®g CaO
![]()
Finally, lets check web the interactive web site for problems of this type.
Our calculations of molar volume earlier in the chapter were limited to gas measurements at STP. What about other temperatures and pressures? To answer that, we need to know how gas volumes change with changes of temperature and pressure, and that behavior is explained by the gas laws.,
Gas Laws
The effect of pressure on volume is described by Boyle’s Law after Robert Boyle who did some of the early measurements that began to establish the scientific approach, and the effect of temperature on volume is described by Charles' Law. Avogadro's hypothesis, that equal volumes of gases at the same temperature and pressure have the same number of molecules is also sometimes called Avogadro's Law. A merger of these three laws gives us an equation that in one statement tells us how the variables pressure, volume, temperature, and quantity all relate to one another for an ideal gas and hence is called the ideal gas law
Boyle’s Law
How many of you have ever pumped up a bicycle tire? You know as you press down on the pump, the air seems to have a spring to it. It pushes back. More precisely, the pressure the air exerts on the piston inside the pump increases when you try to squeeze the air into a smaller volume.4
Who among you have ever used a BB gun? When the gun is cocked, you compress some air to a very small volume. The pressure of that compressed air is high enough to propel a BB at a reasonably high velocity.
Robert Boyle measured the pressure of an enclosed glass as increasing pressure caused its volume to decrease, in order to see how volume, the dependent variable, changed as the pressure, the independent variable, was changed.
Gas pressure can be measured by a barometer. This was a device invented by Torricelli. If you fill a glass tube with mercury and invert it in a pool of mercury, the column of mercury in the tube will fall, creating a vacuum in the tube, and the height of the column of mercury will be supported by the pressure of atmospheric gases on the surface of the mercury. This pressure of the atmosphere represents the weight of a column of air extending from the surface of the earth to the outer limits of the atmosphere.
For an animated description of Torricelli's barometer, see this web site:
We define a standard atmosphere as that amount of pressure which will hold up a column of mercury 76 cm (or 760 mm), and so gas pressure is often measured in terms of “mm of mercury”. That unit is called the torr, after Torricelli.
Why not use water as the liquid in such a barometer? The answer is that the density of water (1.00 g/mL) is much less than the density of mercury (13.6 g/mL), so the column of water that can be held up by atmospheric pressure is about 34 feet high. So it would be cumbersome as a measurement device.
This relationship explains why pumps, working strictly by generating a vacuum, cannot raise the level of water above about 34 feet.
Boyle may have used a device similar to the barometer, except that he would have some gas contained in the closed end, instead of a vacuum. As more mercury is poured into the open end, the gas volume gets smaller. The pressure can be measured by the difference in height of the mercury level in the two sides of the tube, and the volume by the height of the enclosed gas sample.
The web page
http://dbhs.wvusd.k12.ca.us/GasLaw/Gas-Boyle-Data.html
describes Boyle's set-up and some of his experimental data.
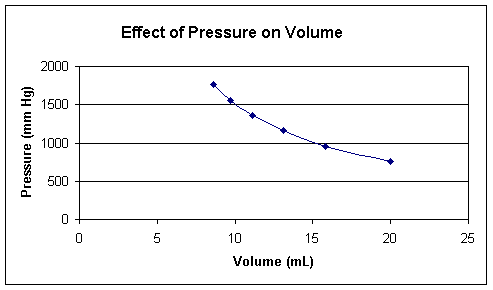
To simplify for the sake of clarification, lets imagine he got the following set of readings:
|
Pressure (mm Hg) |
Volume (mL) |
|
760 |
20 |
|
960 |
15.8 |
|
1160 |
13.1 |
|
1360 |
11.1 |
|
1560 |
9.7 |
|
1760 |
8.6 |
Lets look at how the data looks plotted:
But suppose we play with the data a bit. Instead of plotting P against V directly, what happens if we plot P against 1/V?
|
Pressure (mm Hg) |
Volume (mL) |
1/Volume (mL-1) |
|
760 |
20 |
0.050 |
|
960 |
15.8 |
0.063 |
|
1160 |
13.1 |
0.076 |
|
1360 |
11.1 |
0.090 |
|
1560 |
9.7 |
0.103 |
|
1760 |
8.6 |
0.116 |
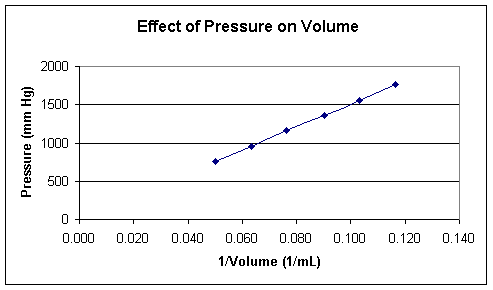
Notice this plot gives a straight line, suggesting that the relationship
![]()
To verify this, try multiplying each P value times the corresponding V value:
|
Pressure (mm Hg) |
Volume (mL) |
PxV |
|
760 |
20 |
15200 |
|
960 |
15.8 |
15168 |
|
1160 |
13.1 |
15196 |
|
1360 |
11.1 |
15096 |
|
1560 |
9.7 |
15132 |
|
1760 |
8.6 |
15136 |
This behavior is easily explained according to the kinetic molecular theory. Pressure of the gas is caused by the collisions of the molecules on the walls of the container. As the volume is decreased, and the number of molecules in a particular volume increases, the number of collisions with the wall increases, resulting in an increase in pressure. One can therefore derive Boyle’s law from considering the simple classical physics of the particles in the gas.
Charles Law
We have already said that the concept of temperature was related to the motion of molecules, and hence to the average kinetic energy of particles in the gas. We would expect, therefore, if the volume were kept constant and the temperature increased, the increasing kinetic energy would lead to more collisions, and therefore an increase in pressure. But suppose the volume were not kept constant, instead the gas were allowed to expand in such a way as to maintain constant pressure? These were the type of experiments carried out by the French physicist Jacques Charles, who was interested in the subject partly because of his interest in hot air ballooning.
If Charles plotted Volume against Celsius temperature, he found a straight line relationship. That is the volume is proportional to the temperature. If this straight line could be extrapolated to low temperatures, that is if the relationship continued to hold for the gas as the temperature got lower and lower, there would reach a point where the volume would become zero. That extrapolated point, then should represent the lowest possible temperature attainable (a temperature in which there was no kinetic energy of the particles). That temperature is called absolute zero. All gases will liquefy before reaching absolute zero, so the extrapolation doesn’t work in practice, but one can theoretically imagine an ideal gas which would not liquefy and which therefore would reach zero volume at absolute zero. (Zero volume, of course, would assume the particles themselves occupied no volume).
This definition of absolute zero, which turns out to be –273.15 oC, is a real limit in temperature, and we have come very close to it with some substances. Some materials take on very different properties as they approach these low temperatures. For the example, the phenomenon of superconductivity in which metals or certain types of compounds conduct electric current with no resistance. Very light gases like hydrogen and helium do not liquefy until close to absolute zero.
So a new temperature scale was suggested, in which zero on the temperature scale represents absolute zero. It is called the Kelvin scale, and the temperatures are represented with the unit K.
Charles Law can then be stated very simply that the volume is proportional to the absolute temperature, or
V = constant T
where T is measured in Kelvin degrees (Celsius degrees + 273).
Ideal gas law
Boyle’s and Charles’ laws can be combined into a generalized law which also takes into account changes associated with changes in the quantity of the gas. If you put more air in your tires, and the temperature and volume are held constant, you note that the pressure increases. It turns out that pressure is directly proportional to the quantity of a gas.
Taking these relationships together gives us what we call the ideal gas law.
PV = nRT
or
PV/nT =R
where R is called the gas constant and has the value of 0.0821 L-atm/mol-oC.
Thus knowing any three of the four variables, P, V, T or n, the fourth is determined and can be calculated.
Review
Now, recall that we had four mathematical equations showing how gases behave with changes in pressure, volume, temperature, or amount.
- Boyle’s Law: PV = constant
- Charles’ Law: V = constant x T
- Avogadro’s Law: V/n = constant
- Ideal Gas Law: PV = nRT,
- where R is the gas constant (0.0821 L-atm/mol-degree K)
The ideal gas law is essentially like a summary collection of the first three laws.
Lets see how we can use these laws to calculate changes in gas behavior when conditions are changed.
Changes in pressure and volume
When we change one of the conditions of a system we are changing the state of the system. So we can consider the conditions before and after the change. So suppose we have a gas at a certain pressure and volume, and we want to know what the new volume will be if we change the pressure? An example might be a tank of gas under pressure, and trying to figure out what volume the gas will occupy when let out of the tank. Let the beginning condition be P1 and V1, and the final condition be P2 and V2, then another way of stating Boyle’s Law is:
![]()
Lets look at number 55, page 182 for example. P1 is 150 atm, V1 is 60.0 L, P2 is 750.0 mm Hg (or torr). What is V2?
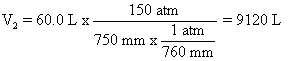
Note in this problem, you need to get the pressure in the same units.
Lets check out the interactive web site once more for practice on these problems..
Changes in Temperature and Volume
Restating Charles’ Law in the two state condition gives:
![]()
So lets look at problem 57, page 182. V1 is 154 mL, T1 is 100 oC, T2 is 10 oC. What is V2. Recognize here that the temperature must be changed to Kelvin by adding 273 oC.
![]()
Let’s check out a problem on the interactive web site as well.
The ideal gas law can be used on a couple of ways. One is to calculate one of the four variables, P, V, T, and n, giving that you know three of them.
Let’s do an example problem. Calculate the pressure in a tank that contains 4.64 mol of CO in a 3.96-L tank at 29 oC. Setting up the problem:
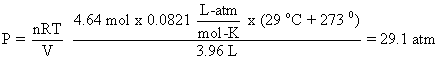
Or, you can use it if multiple changes are being made.
![]()
So, for example, we could look for changes in conditions on volume or on pressure. The equation can be arranged in the following ways, among many others:
![]()
Note here you correct one of the variables always by multiplying by a ratio of the other conditions. And the multiplication factor can be predicted by common sense. There is also an interactive web site to practice these types of problems.
Solutions
Recall that the term "solution" refers to a homogeneous mixture. Air is a solution of several gases. Metal alloys are solutions of two or more metals mixed together, and hence is a solid solution. We are most often dealing with liquid solutions however.
The component in excess in the solution is called the solvent. (For aqueous solutions, water is the solvent). The component present in the minor amount is called the solute. Sodium chloride is the solute in a salt solution, sugar the solute in a sugar solution. (For a mixture of alcohol and water, both liquids, it may become ambiguous which is solvent and which is solute).
We are often interested in expressing the relative amounts of solute and solvent. The properties of a solution might change with added solute, for example and the amount of change depends on the amount of solute. We sometimes use the terms dilute and concentrated to express big differences. Concentrated sulfuric acid is very corrosive (remember the reaction with sugar!), while a very dilute solution of sulfuric acid is no more corrosive than the fluids in our stomach. But these terms are imprecise, and we need a quantitative way of expressing the relative amounts. We refer to the concentration of a solution, and there are a number of ways of expressing concentration.
- amount of solute/amount of solvent---represents a ratio of substances.
- amount of solute/amount of solution--represents a fraction of the whole.
Furthermore, we can express amount of substance either as mass, volume, or number of moles. So these choices can give rise to quite a number of different units for concentration.
Your textbook introduces only three units at this stage:
- percent by volume ((volume of solute/volume of solution) x 100)
- Concentration of gases in the atmosphere, for example.
- percent by mass ((mass of solute/mass of solution) x 100)
- 35.7% H2SO4 for example.
- molar concentration (moles of solute/liter of solution).
Let's look at a few examples:
Question 79, page 183: What is volume percent concentration of:
Question 81, page 183: What is the mass percent concentration of:
Molarity calculations are a bit more complicated. Sometimes you are simply interconverting the units molarity, volume, and moles:
V x M = moles; V = moles/M; M = moles/V
Question 69, page 182: Calculate the molarity of:
Question 75, page 182: What volume of 6.00 M NaOH is required to contain 1.25 mol NaOH?
Sometimes you have to convert from grams to moles or moles to grams in addition:
Question 73 b. How many grams of solute are needed to prepare 10.0 mL of 4.25 M C6H12O6?
You will seldom have a need to do this latter kind of calculation. In preparation for understanding the strength of acid solutions in the next chapter, though, you should be familiar with the first type of interconversions
You can practice that type of interconversion at this interactive web site.
|