

CHM 1020--Chemistry for Liberal Studies--Fall 2000
 |
 |
|
CHM 1020--Chemistry for Liberal Studies--Fall 2000 |
|
Chapter 7 Acids and Bases
As we begin to study chemical reactions, we find that they can be grouped into different categories, depending upon the type of reaction occurring. We won’t have time in the course to cover all types, so we will concentrate on just one of the more common reactions. This type is called an acid-base reaction, in which a hydrogen ion (H+), also known as a proton, is transferred from one chemical species to another.
Many, but not all, chemicals can be classified as either acids or bases. Acids and bases react to form salts and water.
Acids and bases were first recognized and distinguished by some rather simple properties. Acids taste sour. The sour taste of a lemon is a result of the acids in the lemon. Bases taste bitter and feel sort of slippery on the skin.
But I don’t recommend using taste as the analytical method to distinguish between the two classes. Especially since both strong acids and strong bases are very corrosive. They can do extensive chemical damage to living cells. Muriatic acid, which is a strong solution of hydrochloric acid, for example, can dissolve some metals and some minerals, and can be used to clean mortar and other substances off of bricks. Sodium hydroxide, a strong base, is used to dissolve hairs and other debris in the traps of plumbing systems. It is the major constituent of Drano.
On the other hand, weaker acids are tolerated very well by living tissues. Almost every compound in the cells of our body has at least a mild acidic or basic property.
There are a number of natural pigment compounds that change color when exposed to acids and base. One is the substance litmus, which turns a red color in the presence of acid, or blue in the presence of base. Such pigments are referred to as indicators, meaning by their color they can indicate the presence of an acid or a base. These indicators are very useful tools to the chemist for classification purposes. (You have all probably heard of the expression "the litmus test", referring to a definitive test of some sort.)
A particularly interesting indicator is a material extracted from red cabbage. It can change several colors as it is exposed to acids or bases with differing strengths. There are other indicators or mixtures of indicators that change to several different colors, and are referred to as universal indicators.
Lets look at some common every-day substances, using using a universal indicator, to see how they would be classified. The test is more than just a positive or negative, the different color shades can indicate some degrees of the strength of acids.
This degree of strength can be calibrated to a numerical scale with numbers ranging from about 0 to about 15. Low numbers represent acid solution, high numbers represent basic solution, and neutral solutions have an intermediate number which happens to be 7.0 on this scale. The scale is called the pH scale. A bit later we will see where these numbers come from, but for now we can make some qualitative comparisons. pH values 3 or below are considered strongly acidic. A pH from 4-7 is weakly acidic, a ph of 7 is neutral, a pH of 7-10 is weakly basic, and a pH from 11-15 is strongly basic
Demonstration: Includes some standardized tubes with known pH values to use to calibrate the colors of the a universal indicator, then the testing of various familiar substances to classify as acidic or basic.
So what is it chemically that causes these properties for a substance? The Swedish chemist Arrhenius was among the first to recognize that acids and bases behaved as electrolytes, forming ions in aqueous solution. Furthermore, he noted that acids tend to form hydrogen ions (H+) in aqueous solution, while bases tend to form hydroxide ions (OH-) in aqueous solution. These observations, in fact, represent what we call the Arrhenius definition of acids and bases.
Actually, it is not just the presence of a hydrogen ion or hydroxide ion that is important, it is the relative amounts of the two.
In acid solutions, the amount of H+ > the amount of OH-.
In basic solutions, the amount of OH- > the amount of H+.
(In neutral water, we have a very small amount of both ions, but they are in equal amounts being formed by the equation describing the ionization of water:
H2O € H+ + OH-
The double arrow indicates that the reaction is going in both directions. Only a very small fraction, one in every every 5.5 x 108 water molecules in neutral water is dissociated at a time.)
Actually, the description of the proton in water is an over simplification. A proton is the nucleus of a hydrogen atom, a very small particle indeed. in aqueous solution, this proton nudges up against a lone pair in the water molecule, actually forming what we call a hydronium ion (H3O+).
Recall the Lewis dot structure for the hydronium ion:
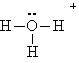
Understand that whenever we specify H+ in aqueous solution, we are always talking about a hydrated proton (and actually there may be more than one water attached to the proton).
Acids then, according to the Arrhenius definition, will be substances that can cause an increase in the hydrogen (or hydronium) ion in water. Lets take HCl, hydrogen chloride, as an example. Recall that the bond between H and Cl is very polar because of the difference in electronegativity between the two atoms. This also means that the bond is relatively weak. When hydrogen chloride dissolves in water, this bond breaks, because the proton is more tightly bound to the lone pair of electrons on a water molecule than the pair of electrons on the chlorine. So the following reaction takes place:
HCl + H2O ® H3O+ + Cl-
The aqueous solution of HCl is called hydrochloric acid. It is a strong acid, because it dissociates completely in water. The strength of the corresponding acid solution depends simply on how much HCl is dissolved. Large amounts produce a very corrosive liquid, muriatic acid. But small amounts can be tolerated. In fact, your stomach contains small amounts of HCl.
So we might expect that acids should contain a hydrogen atom which will dissociate in aqueous solution. But our demonstration with dry ice shows that carbon dioxide as it dissolves in water produces an acid solution. There is no hydrogen in CO2. What is happening?
Carbon dioxide reacts with water to form an acid, carbonic acid:
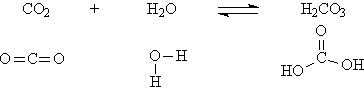
Note that by adding a molecule of water, two OH groups are produced, and the attachment of these hydrogens to the oxygens is weak enough that some of the hydrogen can dissociate.
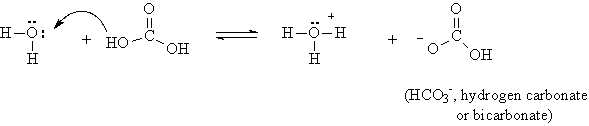
The double arrow indicates that only part of the carbonic acid molecules dissociate, so that carbonic acid is a weak acid.
One can generalize that non-metal oxides will react with water to form acids. Some other examples include:
SO3 + H2O ® H2SO4 (sulfuric acid)
N2O5 + H2O ® 2 HNO3 (nitric acid)
For this reason, non-metal oxides are called acid anhydrides (meaning acids without water).
We said above that Arrhenius defined a base as something that increases the hydroxide (OH-) ions in solution. One obvious class of compounds that can do that are the ionic metal hydroxides. As ionic compounds, they dissociate in aqueous solution. For example, NaOH in water dissociates:
NaOH ® Na+ + OH-
If the hydroxide is completely soluble, the dissociation will be complete, and the solution will be strongly basic. Some hydroxides are not very soluble in water. The amount that dissolves, though, will dissociate into OH- ions, but the solution will be more weakly basic.
In direct contrast to the reaction of non-metal oxides with water, metal oxides react with water to form bases. That is because they form hydroxides. For example:
Na2O + H2O ® 2 NaOH
CaO + H2O ® Ca(OH)2
So we call metal oxides basic anhydrides (meaning bases without water).
Review
Arrhenius definition of an acid and a base:
Acids
Non-metal oxides are acid anhydrides, and though they don't contain hydrogen atoms themselves, they react with water to form oxyacids. For example:
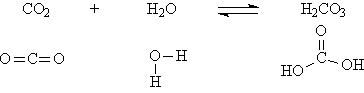
And the carbonic acid formed can then dissociate a proton in water:
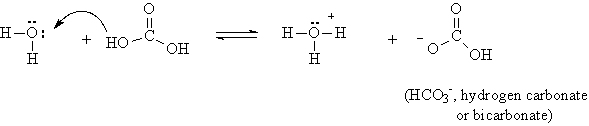
The double arrow indicates that only a portion of the carbonic acid undergoes dissociation. Hence it is called a weak acid.
Other examples include:
Sulfuric acid and nitric acid both happen to be strong acids. This simply means that the OH bonds formed here are weaker than in the case of carbonic acid, and a hydrogen ion dissociates completely. The following equation illustrates the ionization of nitric acid. In this case a one-way arrow is drawn, indicating that dissociation is complete.
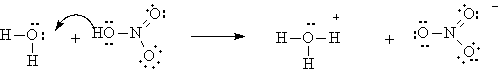
In fact, many acids are simply substances in which one or more hydrogens has been added to the anions we talked about in the last chapter. The names of the acids are related to the names of the anions as well. Lets consider a few:
Acids of monatomic anions (and others ending in ide). The ide becomes ic, and the name is preceded by hydro.
|
Ion |
Name |
Acid |
Name |
|
F- |
fluoride |
HF |
hydrofluoric acid |
|
Cl- |
chloride |
HCl |
hydrochloric acid |
|
Br- |
bromide |
HBr |
hydrobromic acid |
|
I- |
Iodide |
HI |
hydroiodic acid |
|
CN- |
cyanide |
HCN |
hydrocyanic acid |
When we discussed HCl in the chapter on covalent bonding, you may recall we called it hydrogen chloride. There is a subtle distinction here. As a gas, HCl is a distinct molecular species, and it is named like ordinary binary molecular compounds. But in aqueous solution, it dissociates, and we note that dissociation by calling it hydrochloric acid. The same holds for the other compounds in this table. In the absence of water, they are molecular and are named that way (hydrogen fluoride, hydrogen cyanide, etc). But in water they dissociate and are named as acids.
Acids of polyatomic oxy anions. The ate becomes ic, but the name is not preceded by a hydro. Compare the names of the anions from Table 6.2 with the corresponding acids in Table 7.1. Note that the number of negative charges on the anion determines the number of hydrogens in the neutral acid.
|
Ion |
Name |
Acid |
Name |
|
NO3- |
nitrate |
HNO3 |
nitric acid |
|
ClO3- |
chlorate |
HClO3 |
chloric acid |
|
ClO4- |
perchlorate |
HClO4 |
perchloric acid |
|
CH3CO2-- |
acetate |
CH3CO2H |
acetic acid |
|
SO42- |
sulfate |
H2SO4 |
sulfuric acid |
|
PO33- |
phosphate |
H3PO4 |
phosphoric acid |
For acids formed from oxyanions whose names end in ite, change the ite to ous rather than ic.
|
Ion |
Name |
Acid |
Name |
|
NO2- |
nitrite |
HNO2 |
nitrous acid |
|
SO32- |
sulfite |
H2SO3 |
sulfurous acid |
|
ClO2- |
chlorite |
HClO2 |
chlorous acid |
|
ClO- |
hypochlorite |
HClO |
hypochlorous acid |
Strong acids are acids that dissociate completely in water. There are only seven common ones: HCl, HBr, HI, HClO3, HClO4, HNO3, and H2SO4. The rest dissociate only partly in aqueous solution and are classified as weak acids. Of course there are varying degrees of weakness as we shall see later.
Bases
In contrast to non-metal oxides, metal oxides are basic anhydrides. They react with water to form ionic hydroxides, which then dissociate in water to give OH- ions. For example:
Most Arrhenius bases are metal hydroxides (or form metal hydroxides) on reaction with water. Most metal hydroxides would be considered strong bases because they dissociate completely when dissolved in water, but some are not very soluble in water and therefore will not produce a solution with very high concentration of hydroxide ions. Mg(OH)2, for example, is not very soluble. So a suspension of this salt is not very corrosive, and in fact is the ingredient in milk of magnesia.
Why do metal oxides produce hydroxides, and non-metal oxides produce protons? When the oxides react with water, an OH is attached to the atom. It is the differing electronegativities (E.N.) of the metal and non-metal that determines whether the OH group dissociates as an ion or the O-H bond dissociates to form H+. The metal is less electronegative than hydrogen, so the electrons on the oxygen are pulled away from the metal and more toward the hydrogen, making the metal-oxide bond more polar and thus dissociating into ions. A non-metal is more electronegative than hydrogen, pulling the electrons around oxygen more toward the non-metal and away from the hydrogen, making the O-H bond the more polar of the two, and this is the bond which dissociates to form H+. This behavior is described in the following diagram:
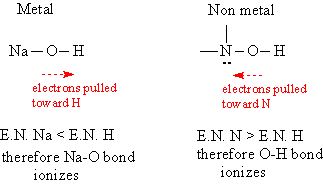
Other bases
Some Arrhenius bases produce hydroxide ions in water by reacting with the water. An example is ammonia:
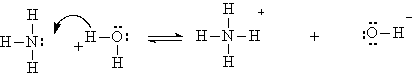
Ammonia is a weak base, because the reaction does not proceed very far to the right, and hence only a small number of OH- ions are formed. This partial reaction is represented by the double arrow.
Neutralization Reactions
If acids form H+ ions in water, and bases form OH- ions in water, then it stands to reason that when they are mixed, the hydrogen ions would react with the hydroxide ions to form water:
H+ + OH- ® H2O
or, using the more correct representation of the hydrogen ion:
H3O+ + OH- ® 2 H2O
(Note this is the reverse of the dissociation of water which we discussed earlier, but pointed out that only a very small amount of ions can be present at the same time.)
So writing a reaction between an acid and a base involves reacting the H from the acid with the OH from the base to form water, and the other product is a salt, which consists of the metal (from the basic metal hydroxide) and the anion from the acid. Following are some examples:
In these cases, one can clearly see the H and OH in the reactants that react to form water. But the reaction of the base NH3 with HCl in aqueous solution bit more subtle:
NH3 + HCl ® NH4Cl
You can see the salt, but what happened to the water? Well, you could consider the reaction as occurring in two parts:
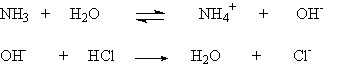
The first step is the incomplete reaction of ammonia with water to form ammonium hydroxide. (Note the double arrow, indicating that this reaction only goes part way). The second is the complete reaction of hydroxide with HCl, removing the hydroxide formed in the first step, which is replaced as it is removed with more ammonia reacting with water. (Note the unidirectional arrow). So the water formed in the second step is just replacing that used in the first step.
But suppose this reaction didn’t occur in water? In fact, gaseous NH3 and gaseous HCl will react in exactly the same overall way:
NH3 (gas) + HCl (gas) ® NH4Cl (solid)
This is still considered an acid-base reaction. Such reactions are more easily analyzed using a more encompassing definition of an acid and a base, one called the Bronsted-Lowry definition:
An acid is a proton donor. A base is a proton acceptor.
So in the above reaction, NH3 is a base not because it can form OH- in solution, but because it can accept a proton to form NH4+. HCl is a base because it can donate a proton. In this view, the passage of a proton forms a new pair of acids and bases!
Consider that the reaction is reversible:
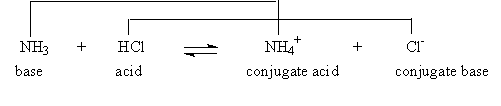
So an acid-base reaction is just the passing back and forth of a proton. A species with a proton it can donate is an acid. After it donates the proton, it becomes a base, because it can accept the proton back. When the base in a reaction accepts a proton, it becomes an acid with a proton it can donate. Sometimes the same substance can be acting as both an acid and a base at the same time, which is the case in the very slight dissociation of water we discussed earlier.
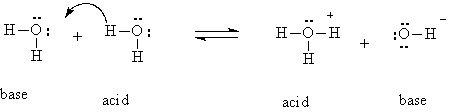
The strength of an acid solution is determined by the amount of H+ in the solution. The strength of a basic solution is determined by the amount of OH- in solution. We express the quantity of a solute in a solvent as a concentration.
![]()
There are several different ways of expressing both the quantity of solute and the quantity of the solvent or solution, so there are several kinds of concentration units. For describing acid and base concentrations, we express the solute in moles and the amount of solution as a volume, expressed in liters. So the concentration unit is moles/liter (or mol/L), which we abbreviate molar, M. So a solution with a concentration of 0.10 M has 0.10 moles of solute in 1 liter of solution. (Not solvent—total solution). This concentration makes it very convenient to measure quantities of solutes by simply measuring the volume of the solution, rather than having to weigh something. And one can then easily do calculations about relative amounts of solutions that participate in chemical reactions.
Let's concentrate, though on expressing concentration of H+ and OH-.
Recall that H+ is really a hydrated proton, and could also be represented as H3O+. What is the maximum concentration we might expect to have of hydrogen ion in aqueous solution?
Clearly there can’t be more H3O+ species than there are water molecules to begin with. So we might first ask, what is the molar concentration of water, i.e. [H2O]? The square brackets, [ ] signify we are talking about the molar concentration, or M.
1000 g/L x 1 mol/18.0 g = 55.6 mol/L or 55.6 M.
[H3O+] must be less than this because there must be some kind of anion in the solution to neutralize the positive charge. Therefore the maximum acid concentration is probably somewhere about 20 M.
What would be the minimum concentration of H+? At first thought, you might be tempted to say zero, but that is not the case because of the following reaction that water undergoes:
H2O + H2O ® H3O+ + OH-
This reaction represents the proton from one water molecule that is hydrogen bonded to a neighbor actually being transferred to the neighbor. This reaction is more easily explained using the Bronsted-Lowry definition of an acid mentioned in the last lecture. One of the water molecules is acting as an acid (a proton donor), and the other as a base (a proton acceptor). After the transfer the conjugate base of the donor water is formed (OH-), and the conjugate acid of the acceptor water is formed (H3O+). The proton can be transferred back, so the reaction can actually occur in both directions, as illustrated in the following equation:
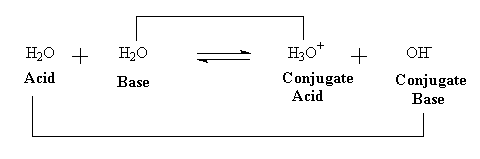
When the rate of the forward reaction is equal to the rate of the reverse reaction, there is no further increase in hydrogen ions and hydroxide ions, and the solution is in what we call a state of dynamic equilibrium.
Equilibrium is a term applied to a system when there is no apparent change in the composition of the system with time. There are two types of equilibrium: static and dynamic.
In a static equilibrium there is no change because nothing is happening. In a dynamic equilibrium, there is no net change because two things are happening in the opposite direction at equal rates. Imagine yourself paddling a kayak upstream, where you are just able to paddle as rapidly as the stream is flowing. Your position remains stationary relative to the bank of the stream, just next to a car parked on the bank. The car is not moving, and is in a state of static equilibrium. Your kayak is also stationary relative to the bank, but it is in a state of dynamic equilibrium. Another example would be running on a treadmill, where you have no net forward motion because your forward motion is counteracted by the motion of the tread.
Dynamic equilibria in chemical reactions can be expressed quantitatively by what is called an equilibrium constant. In the case of the reaction stated above, the position of the equilibrium is expressed by the relationship that;
[H3O+][OH-] = a constant, Kw, which = 10-14.
This equation says that the product of the concentration of hydrogen ion and the concentration of hydroxide ion remains constant. If one goes up, the other must go down.
In a neutral solution, [H3O+] = [OH-]. Let’s call that value x.
Then from the relationship above:
[H3O+] can be decreased further by increasing the hydroxide concentration. But just as there is a maximum hydrogen ion concentration, limited by the number of water molecules, there is a maximum hydroxide concentration, also in the region of about 20 M.
If [OH-] were 20 M, what would the [H3O+] be?
In other words, hydrogen ion concentration can range from a high of near 20 M to a low of around 5 x 10-16. This is a range of almost seventeen orders of magnitude (powers of ten).
- When [H+] > [OH-], the solution is acidic.
- When [OH-] > [H+], the solution is basic.
Following are some possible combinations:
|
[H+] M |
[OH-] M |
|
|
10 |
10-15 |
Acidic |
|
10-2 |
10-12 |
|
|
10-4 |
10-10 |
|
|
10-7 |
10-7 |
Neutral |
|
10-10 |
10-4 |
Basic |
|
10-12 |
10-2 |
|
|
10-15 |
10 |
So you are told a solution has a concentration of 3.5 x 10-4 [H+].
It is inconvenient to express such a broad concentration range with a linear scale. It is impossible to get more than three orders of magnitude on one graph, and even that is difficult. For this reason, chemists revert to a logarithmic scale. The logarithm of a number is the power of ten equivalent to that number. For example:
|
n |
log n |
|
|
100 |
102 |
2 |
|
1000 |
103 |
3 |
|
10 |
101 |
1 |
|
1 |
100 |
0 |
|
0.1 |
10-1 |
-1 |
|
0.01 |
10-2 |
-2 |
|
0.001 |
10-3 |
-3 |
These numbers are for even powers of ten. What about other numbers? By entering the numbers on your calculator, and punching the logarithm button, you will find that:
Now, use your calculator for the following series of numbers:
Remember that you multiply powers of ten by adding exponents:
10a x 10b = 10(a+b)
It follows that log (10a x 10b) = log (10(a+b)) = a + b
So:
log 300 = log (3.0 x 102) = log 3.0 + log 102 = 0.48 + 2.0 = 2.48
And:
log 0.03 = log(3.0x10-2) = log 3.0 + log 10-2 = 0.48 – 2.0 = -1.52
A logarithm consists of two parts, related to the two parts of a number expressed in exponential notation.:
The mantissa, which is the part of the logarithm after the decimal, refers to the numerical part of the exponential notation.
The characteristic, which is the number before the decimal, refers to the power of ten in the exponential notation.
In the logarithm 2.48, 0.48 is the mantissa, referring to 3.0, and 2 is the characteristic, referring to 102.
Back to hydrogen ion concentration
We define a logarithmic scale of hydrogen concentration as follows:
pH = -log [H+]
So, expressing [H+] as pH in the earlier table;
|
[H+] M |
pH |
[OH-] M |
pOH |
|
|
10 |
-1 |
10-15 |
15 |
Acidic |
|
10-2 |
2 |
10-12 |
12 |
|
|
10-4 |
4 |
10-10 |
10 |
|
|
10-7 |
7 |
10-7 |
7 |
Neutral |
|
10-10 |
10 |
10-4 |
4 |
Basic |
|
10-12 |
12 |
10-2 |
2 |
|
|
10-15 |
15 |
10 |
-1 |
Notice that as acidic strength, and hydrogen ion concentration increase, pH decreases. (Therefore, the anecdote about a legislator trying to pass a law to reduce pH to zero because of the problem of acids shows a gross misunderstanding of the meaning of this term.)
In dealing with [OH-] concentrations, we define similarly
pOH = -log[OH-]
So lets go back and insert pOH values in the table.
And we note that pH + pOH = 14, so we can easily convert from one to another:
pH = 14 - pOH, or pOH = 14 - pH
Lets look at the problems in the back of the chapter (p. 193, starting at 49.0
Closing comments
Just a few words about the last three sections of the chapter. We have already talked about acid rain as coming from the acidic non-metal oxides of sulfur and nitrogen that are produced in combustion of coal and other fuels. We also mentioned milk of magnesia, a suspension of magnesium hydroxide, as being basic and being used to help neutralize excess stomach acid. Actually there are many things on the market for that purpose, as you can see in table 7.5. Tums, for example, is just CaCO3, which is really just limestone. Limestone is basic. While it won't dissolve in neutral water, it will dissolve in acid solution as a result of the following reaction:
CaCO3 + 2 HCl ® CaCl2 + H2CO3
The H2CO3, carbonic acid, can decompose into CO2 and water, the reverse of the process we demonstrated with the dry ice last lecture. The direction the reaction goes depends on the acidity or basicity of the solution.
Limestone also can be decomposed into CO2 directly by heating:
CaCO3 ® CaO + CO2
CaO, known as lime is produced to the extent of about 17 billion kg per year in the US. It is used commercially as a base to treat acid soil in agriculture because it reacts with water to form Ca(OH)2.
The strong acids HCl and H2SO4 and the strong base NaOH have many industrial uses and are also made in great quantity. These are very corrosive materials, especially in the near pure state or in high concentrations in water, so you should handle with extreme caution if you handle them at all.
|