

CHM 1020--Chemistry for Liberal Studies--Fall 2000
 |
 |
|
CHM 1020--Chemistry for Liberal Studies--Fall 2000 |
|
Chapter 9—Organic Chemistry
At one time organic compounds were thought to have originated from living organisms, while chemicals not made by living tissue were considered inorganic compounds. In 1828 this distinction was removed when a German chemist Friedrich Wohler made urea (an organic compound, produced in the urine of animals) from ammonium cyanate, a purely inorganic compound.
It still took most of the 19th century for scientists to accept that there is nothing particularly special about the chemicals in living organisms and the chemicals made in other ways. There is no vital force that distinguishes one from the other.
Today, we classify organic chemistry as the chemistry of carbon containing compounds. Carbon has a very special, and unique, property among the elements: it can form bonds with other carbon atoms producing very large molecules of many carbon atoms, and it also forms stable bonds with hydrogen, oxygen and nitrogen.
As molecules get bigger, and the number of atoms increases, so does the way in which the atoms can be attached to each other increase. Astronomically. Though maybe about 20 million compounds have been isolated and identified—and over 90% of them are carbon-containing—the number of possible compounds is essentially infinite. If you could imagine having just one molecule of every possible conceivable structure, the sample would take up a volume far larger than the volume of the known universe.
Because of the enormous variety of structures possible for carbon compounds, the nomenclature gets more complicated than the simple nomenclature we discussed earlier for covalent compounds. Whenever we have such a large variety of possibilities, we must simplify by trying to classify compounds according to certain typologies, and develop a systematic nomenclature for the classification.
The simplest organic compounds contain only carbon and hydrogen. Hence they are called hydrocarbons. Hydrocarbons, it turn, can be subdivided into classes according to some structural characteristics:
| Class | Property |
|
alkane |
contains only C-C and C-H single bonds |
|
alkene |
contains at least one C=C double bond |
|
alkyne |
contains at least one triple bond |
|
cycloalkane |
contains a ring of C atoms |
|
cycloalkenes |
contains a ring of C atoms and at least one double bond |
|
cycloalkynes |
contains a ring of C atoms and at least one triple bond |
|
aromatic hydrocarbons |
contains a ring of C atoms with double bond resonance structures |
You can see how quickly this classification scheme grows and multiplies when you consider having all possible combinations of ring structures in combination with double bonds inside and outside the ring.
Recall that a molecular formula gives the number and kind of atoms in a molecule, while a structural formula gives the way in which atoms are attached to each other. Recall also that molecules are three dimensional objects, and the VSEPR theory helps explain how atoms are geometrically spaced around one-another. In particular, remember that carbon tends to form 4 bonds, and these bonds are arranged in a tetrahedral structure around the carbon atom.
Lets first look at some simple linear alkanes (called normal alkanes) and several ways to represent the formula and the structure.
|
Alkane |
|
|
|
|
methane |
CH4 |
CH4 |
|
|
ethane |
C2H6 |
CH3CH3 |
|
|
propane |
C3H8 |
CH3CH2CH3 |
|
|
butane |
C4H10 |
CH3CH2CH2CH3 |
|
|
pentane |
C5H12 |
CH3CH2CH2CH2CH3 |
|
But lets take a closer look at the geometrical structures:
| Alkane | Geometrical Drawing | Molecular Model |
| methane | |
|
| ethane | 
|
|
| propane | 
|
|
| butane | 
|
|
| pentane | 
|
|
You can see these and some other molecules in 3D at this web site. Make sure you have Chemscape Chime installed on your computer, and click on the PDB link for the structure you are interested in.
Note that Lewis structures make it difficult to compare two structures with the same formula to tell if they are different or not. For example, the following three structures for butane are equivalent
|
|
|
|
But the following structure is different:

In the first three structures, you have two "central" carbon atoms, each connected to two carbons and two hydrogens, and two "terminal" carbons, each connected to one carbon and three hydrogens. In the fourth structure you have one central carbon, connected to three carbons and one hydrogen, and three "terminal" carbons, each connected to one carbon and three hydrogens. Thus the order of attachment is different, and these compounds are isomers of each other. One is called n-butane (n stands for normal), the other is iso-butane.
(Remember that isomers are compounds with the same molecular formula, C4H10 in this case, but different structural formulas.)
As the number of carbon atoms increase, the number of isomers increases dramatically. There are three isomers for C5H12 (see if you can draw them), There are five isomers of hexane, nine isomers of heptane, eighteen isomers of octane and 35 isomers of nonane. At the size of C14H30, there are 1858 possible isomers. So you can see that even for compounds as simple as alkanes, as the molecules get larger, the number of different possible structures becomes amost astronomical.
Note the general formula for alkanes is CnH2n+2.
It often gets cumbersome even to write the condensed structural formula of large hydrocarbons, so simplified method is used called a stick formula. In the stick formula, a line is drawn to represent a C-C bond. Each place where the lines connect represents a C atom, and hydrogens are not drawn, with the understanding that each C atom has enough hydrogens attached to it to form a total of 4 bonds. A carbon attached to one other carbon will have three hydrogens, a carbon attached to two other carbons will have two hydrogens, a carbon attached to three other carbons will have one hydrogen, and a carbon attached to four other carbons will have no hydrogens. Following is an example of such a stick formula, showing what it represents:

Properties:
Note in table 9.3 the melting and boiling points of a series of alkanes. Both increase as molecular weight increases. Methane is also known as "marsh gas" as it is produced by some microorganisms in swamps. Propane is a gas but can be liquefied under pressure-that is how it is stored in tanks for use in your backyard grill. Butane is liquefied under pressure in cigarette lighters. Pentane and above are liquids at room temperature. Gasoline contains hydrocarbons in the molecular weight range of octane. Above 20 carbon atoms the substances are solids at room temperature. Asphalt used for paving on roads contains very large molecular weight hydrocarbons.
The intermolecular forces between hydrocarbons are strictly dispersion forces caused by the instantaneous dipoles created as electrons fluctuate around the molecule. An instantaneous dipole in one molecule induces one in its neighbor. These are the weakest intermolecular forces (refer back to Chapter 5), but they do increase with molecular weight as the molecules get larger, and the electron clouds get larger and more diffuse.
Cycloalkanes
Carbon atoms can form rings. For example:

Alkenes
Contain one or more carbon-carbon double bonds.
Alkynes
Contain one or more carbon-carbon triple bonds.
Alkenes and alkynes are more reactive than alkanes:
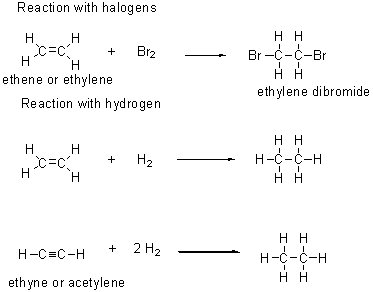
Aromatic compounds
These involve ring structures with alternating double bonds where resonance structures are possible:

Other atoms besides C and H
Halogen Derivatives
One or more hydrogen atoms of a hydrocarbon can be substituted by a halogen. For example, compare:

Some halogen derivatives that have been in the news recently are compounds used as gases in refrigeration and air conditioning systems. They have the proper physical properties to work in a heat exchanger, and are much more inert and innocuous chemically than ammonia, which was first used for this purpose.

But it turns out that these compounds persist in the environment and ultimately diffuse to the upper atmosphere. There interaction with UV light from the sun breaks the carbon-chlorine bond, forming free radicals, which participate in a reaction that leads to destruction of the ozone that provides a protective shield around us. As a result of the studies showing this relationship, production of these compounds has been banned, and new substitutes have been sought for air conditioners.
Another familiar compound involving halogens is a polymer (large molecule) containing carbon chains attached to fluorine atoms rather than hydrogen. This substance is called teflon. The carbon-fluorine bond is very stable, and the molecule overall is non-polar and doesn't adhere to many things, hence it is used as a coating in frying pans and other cooking utensils.

Compounds with oxygen, nitrogen, or both
Other compounds contain one or more oxygen atoms, one or more nitrogen atoms, or some combination. Any particular collection of these atoms is referred to as a functional group, and part of understanding the chemistry of organic compounds is learning the properties and reactions of particular functional groups. The reactions of a functional group will be similar even if the rest of the molecule is vastly different.
The simplest functional group with oxygen is to substitute a H with an OH, which forms an alcohol:
Look at Table 9.4 to get an appreciation of the many different types of functional groups and their names. Then note on table 9.5 that the hydrocarbon part of a molecule containing a functional group is named in a way consistent with the "parent" hydrocarbon in which a hydrogen was substituted for the functional group. These examples should just serve to illustrate for you how numerous the many different structures and structure types are that one encounters in studying the chemistry of organic compounds.
Postscript:
We have only scratched the surface of chemistry, particularly as it pertains to the rich variety of organic chemicals which include the chemicals that make up our bodies. I would encourage you to read some of the subsequent chapters in the book to give you some insight into the variety of compounds you encounter every day, and how chemicals impact on your life.
|