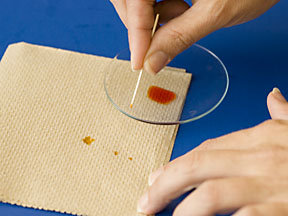
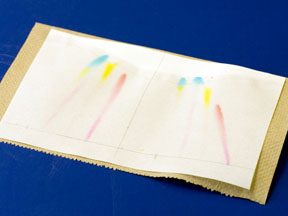
SAFETY NOTES: The solvent used in the chromatography tank generates significant fumes that can be corrosive to contact len material. No contacts should be worn during this lab experiment. Persons with asthma should notify their TA so that they can work at the hood to avoid any irritation that the fumes might cause.
GENERAL INSTRUCTIONS: Each student will generate their own chromatogram.