SAFETY NOTES: Vinegar contains acetic acid. While not a strong acid, it will still cause irritation if gotten in the eyes. The Alka Seltzer can react violently enough to "spray" some acid out of the beakers so wear goggles at all times.
GENERAL INSTRUCTIONS: Each bench will work as a team to complete this experiment with each student completing two of the eight runs required.
| Procedure: |
|
 |
Weigh a clean, dry 100 mL beaker and small watch glass to the closest milligram, (0.001g). (Note: When weighing an object always record all digits displayed, as the number of digits indicates the accuracy of the digital scale.) Record the weight in your lab notebook. |
 |
Using a graduated cylinder, place 35 mL of deionized (DI) water in the 100 mL beaker you weighed |
 |
Re-weigh the beaker with the water. Make sure the outside of the beaker is dry and use Kimwipes to remove any fingerprints or dust etc. Record the weight in your lab notebook. |
|
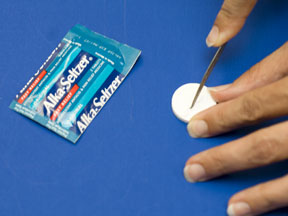
Collect an Alka-Seltzer tablet from the front counter. Break it in half and weigh each half carefully to the closest milligram. |
 |
Add one of the half tablets to the beaker with the water in it. Place the pre-weighed watch glass over the top of the beaker (in an upside down or dome side up orientation) to deflect any splatter. Swirl the contents to ensure the tablet is dissolved completely into solution. |
 |
After the reaction has stopped (stopped bubbling), record the mass of the beaker, its solution and the watch glass. The watch glass must be weighed separately from the beaker and solution. Remove it carefully allowing any accumulated solution to drip back into the beaker. Turn it over quickly to catch any remaining solution. The solution should then pool in the middle of the watch glass. Record the weights in your lab notebook. |
 |
Empty the contents of the beaker down the sink and rinse the beaker with DI water. Dry the beaker and watch glass thoroughly using paper towels and Kimwipes. |
 |
Using a graduated cylinder, place 5 mL of Vinegar in the 100 mL beaker. Then, use a graduated cylinder to add deionized (DI) water until the volume of solution in the beaker is 35 mL. |
 |
Reweigh the beaker with the solution. Make sure the outside of the beaker is dry and use Kimwipes to remove any fingerprints or dust etc. Record the weight in your lab notebook |
 |
Add the second half tablet to the beaker with the water in it and cap with the watch glass (make sure the watch glass is dome side up) as before. Swirl the contents to ensure the tablet is dissolved completely into solution. |
 |
After the reaction has stopped, reweigh the beaker, its solution and the watch glass. The watch glass must be weighed separately from the beaker and solution. Remove it carefully allowing any accumulated solution to drip back into the beaker. Turn it over quickly to catch any remaining solution. The solution should then pool in the middle of the watch glass. Record the weights in your lab notebook.
Repeat steps 7–12 for 10, 15, 20, 25, 30, and 35 mL of vinegar. |
| |
|
| |
|









