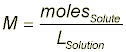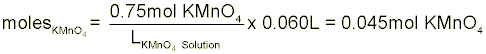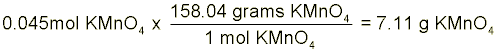|
Perhaps one of the most important aspects to understanding chemistry is the balanced chemical equation. En route to comprehending just how valuable a chemical equation may be, you first need to appreciate Lavoisier’s Law of Conservation of Mass. Recall from the purpose, that in its simplest terms, the law of conservation of mass states that matter can be neither created nor destroyed. Therefore, because matter is composed of atoms that are unchanged in a chemical reaction, mass must be conserved, as well as the number and types of atoms in a chemical equation. The Reaction Redox
In our first reaction, you can observe that the zinc metal is going from a charge of “0” to “+2”. In order to accomplish this feat, the zinc metal has to lose two electrons, or be oxidized. In the same manner, Cu2+ gains two electrons to form Cu0 and is therefore reduced. In the second ‘mini’ reaction, the hydrogen has an oxidation number of +1 on the reactants side and 0 on the product side. Is it oxidized or reduced? [Answer] Net Reaction
Solution Preparation Instead of preparing your exact solution for you, let’s pretend that we have to make 60-mL of a 0.75-M solution of potassium permanganate (KMnO4) in 3.0-M HCl. The first step in this process is to collect ~60-mL of the 3.0-M HCl in your 100-mL graduated cylinder. With the HCl obtained, we have to calculate just how many grams of KMnO4 we will need to make a 0.75-M solution. In order to do this, we first have to find the number of moles in our solution and then multiply by the molecular weight of KMnO4. Molarity is defined as the number of moles of solute divided by the liters of solution. Note that we said liters of solution not just liters of solvent. This means that the total solution volume (including solute volume) is in the denominator.
We will use this same type of process to produce the solution needed to perform this week's experiment. Volume of H2 Evolved As stated above, the reaction we will be observing produces hydrogen gas. In order to determine that the mass of reactants and products has been conserved, we need a method by which we can collect and determine the mass of this hydrogen gas. In order to accomplish this task successfully, you will be given a side-armed Erlenmeyer flask: and a balloon. By sealing the end of the balloon to side-arm, all of the gas released from the reaction will fill up the balloon. We can then measure the volume of the balloon by measuring its radius (r), and height (h) if necessary, and plugging them into one of the following equations:
or Assuming that the balloon’s walls are negligible in volume, we can say that the volume of the balloon is equal to the volume of the hydrogen gas produced. Finally with this volume, you can calculate the mass of the hydrogen gas by using a form of the ideal gas law. We won't go into the details of this equation now, but simply show you how to use the equation to calculate the mass of the hydrogen gas. The Ideal gas equation is PV = nRT, where P is the room pressure which you will read from the barometer in the lab, V is the volume calculated using the equations above, n is the amount of gas in moles (what we are solving for), R is a constant with a value of 0.08206 L.atm/mol.K and T is the room temperature which is also read from the thermometer in the laboratory. Here is a practice problem: Assume that the room temperature is 25 oC (298K) and the room pressure is 760 mmHg (1 atm), if the diameter of your spherical balloon is 4.2 cm, what is the mass of your hydrogen gas? [Answer]
|
 |
Conservation of Copper