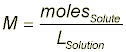|
The purpose should be several well constructed sentences regarding what your experiment is designed to accomplish. These sentences should include both concepts and techniques. The procedure section should reference the lab manual and include any changes made to the procedure in the lab manual. The data section should include a table with the following information in some type of organized format. For before the reaction takes place include a) mass of 250mL side-arm flask, b) mass of 4 or 5 pieces of solid zinc metal on Kimwipe, c) mass of 1 Kimwipe, d) mass of 250mL side-arm flask with CuSO4 acidic solution, e) mass of CuSO4 acidic solution alone, f) mass of Zn metal alone and g) total mass before the reaction. For after the reaction takes place include a) mass of metal pieces on Kimwipe, b) mass of 250 mL side arm flask with CuSO4 acidic solution, c) mass of solution alone, d) mass of metal pieces alone, e) total mass after the reaction, f) volume of the balloon and g) calculated mass of H2 gas alone. Finally calculate the mass % recovered after the reaction. A majority of the information for the tables above can be copied right out of your lab notebook into the report. The values that need to be calculated can be done using the following formulas: Mass of CuSO4 acidic solution alone = (Mass of 250mL side-arm flask with CuSO4 acidic solution) – (Mass of 250mL side-arm flask) Mass of Zn Metal alone = (Mass of 4 or 5 pieces of solid zinc metal on Kimwipe) – (mass of 1 Kimwipe) Total mass of reaction before = (Mass of CuSO4 Solution Alone) + (Mass of Zn Metal Alone) Volume of Balloon= 4/3 pr3 for a sphere or pr2h for a cylinder Calculated Mass of H2 gas along (g) = 8.244 X 10-2 x (volume in L) Total mass of reaction after = (Mass of CuSO4 Solution Alone) + (Mass of Zn Metal Alone) + (Mass of H2)
The data section should also include all observations made during the experiment and the exact concentration or Molarity of CuSO4 solution using the following formula: (see the pre-lab if you have any questions about this calculation).
For the calculation section show one sample calculation for each calculation done to complete the experiment. Make sure that this section is typed or written in ink and that each calculation is labeled as to what is being calculated and why. The conclusion for this experiment should be in paragraph format. State total mass before the reaction and the total mass after the reaction, then discuss the mass percent recovered. In this discussion include if the mass recovered supports the law of conservation of mass or not. Be sure to explain what ever conclusion you reach and provide plenty of support for your conclusion. Also discuss the amount of H2 the reaction produced. Finally include a discussion of possible errors in the experiment and how they might have affected the results. Answer the following questions: 1) Why is the acid in the reaction necessary? Why can't the solution of copper II sulfate simply be made with water? 2) Explain how the Law of Conservation of Mass is the basis for all of stoichiometry.
|
 |
Conservation of Copper