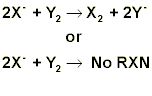Introduction One of the most useful things about studying chemistry is discovering the amount of valuable information contained in the periodic table. Many of the periodic trends of the elements are discussed in Chapters 8 and 9 of your textbook, specifically sections 8.6, 8.7, 9.8, and 9.9. In today’s experiment, the relative electronegativites of the Group 7A elements—commonly referred to as halogens—and their compounds will be exploited through their solubility properties. Electronegativity Electronegativity (χ) is a measure of the ability a particular atom in a molecule to attract an electron to itself. On the periodic table, the most electronegative element is fluorine with a value of 4.0 followed by oxygen with a value of 3.5. Observing Figure 9.14 in your textbook, you can see that a majority of the elements actually possess values to describe their electronegativity. Making you memorize the electronegativity value for each element is unnecessary. This is because the arrangement of the periodic table allows for trends in properties like electronegativity, ionization energy, atomic size, and electron affinity to be easily identified and utilized. The generalized trend for electronegativity is shown below:
Electronegativity and Ions By now you should be familiar with the fact that non-metals generally form anions (negatively charged ions). What you may not know is that anion stability is strongly affected by the electronegativity of the atom upon which the negative charge rests, i.e., the ability of the atom to stabilize additional electrons. Generally, the more electronegative elements are much more stable in anionic form than less electronegative elements. Halides, Halogens, and their Reactions Group 7A elements are diatomic in nature and are called halogens.Some examples include F2, Cl2, Br2, and I2. Group 7A elements that have become ions by gaining an extra electron are called halides. Some examples include F-, Cl-, Br-, and I-. Also note that anions, such as halides, when paired with a cation are ionic compounds not covalent. Therefore, everyday common table salt is an ionic compound, composed of the sodium cation and the chloride anion, called sodium chloride, NaCl.
If a halide solution, let’s call it X-, is added to a solution of a different halogen, let’s say Y2, then there are two possibilities. If element Y is more electronegative than element X, then Y2 will take the electron from X- and leave X 2 as shown below. On the other hand, if Y2 is less electronegative than X-, then no reaction will take place, and Y2 remains as the halogen.
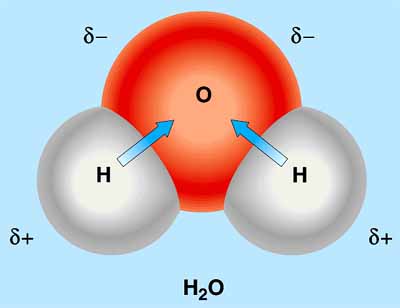
Halides, Halogens, and Solvents In this experiment, the properties of two solvents, water (H2O) and hexane (C6H14), will be useful in sorting out whether a reaction occurs or not. This is because while water is a polar solvent, hexane is not. This means that polar or ionic compounds will reside in the water layer , while non-polar compounds will exist in the hexane layer. Always remember that "like dissolves like". As a polar solvent, water will solvate polar or ionic molecules. A polar molecule is a molecule that has a dipole. A dipole is formed in a molecule when the electrons in that molecule are not shared equally. This unequal sharing of electron density is created by the differences in the electronegativities of the atoms in the molecule. The electrons concentrate around the more electronegative atom(s) and create a partial negative charge around those atoms. The other atoms in the molecule are now somewhat naked of electrons and take on a partial positive charge.
Continuing, a diatomic molecule, like the water molecule above, is polar if the two atoms have different electronegativities. If the electronegativities are exactly the same , as in a halogen, the molecule is non-polar. We encourage you to look back now at the equations just presented and identify which of the halogens and halides are ionic and which are non-ionic. Nonpolar solvents like the organic solvent hexane solvate Nonpolar molecules. Since water is polar and hexane is nonpolar, the two do not mix, and when added together, two distinct, colorless layers are formed. The denser liquid, water, is on the bottom. If colored compounds are added to a test tube containing water and hexane, the polarity of the compounds can be determined. Specifically, if the compounds added are nonpolar, they will color the hexane layer, but if the added compounds are polar, the water layer will be colored. The Experiment For the reaction postulated earlier if Y2 is green compound and is placed in a tube with water (H2O) and hexane (C6H14) , then the hexane layer will become green because the nonpolar Y2 will move to the nonpolar solvent. If we now add some X- to the same tube, as the reaction is allowed to proceed, the green slowly disappears from the hexane layer because Y2 molecules are reacting and moving into the water layer.. The hexane will then take on the color of X 2. On the other hand, if X is more electronegative than Y, the more electronegative atom already has the electron. In other words, no reaction would occur, and the hexane layer would remain green, the color of Y2. In summary, whenever a color change occurs you should be aware that a reaction is most likely taking place. Three halogens in aqueous solution will be available in your lab. They are chlorine, bromine, and iodine. Specifically, each of these halogens has a distinctly different color in hexane, therefore, by observing the color of the hexane layer, the identity of which halogen is present can be determined. When you add a halide solution to each of the halogens, by observing the color of the hexane layer, you will be able to tell if a reaction has occurred or not.
|
 |
Halogen Reactions