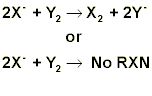The purpose should be several sentences stating both concepts and techniques covered in this experiment. The procedure section should reference the lab manual and note any changes made to the experiment. For this experiment the procedure section should also include if you tested potassium salts or sodium salts, and whose data you used for the other set. The data section should include two tables. The first table should be for the solubility tests. This table should include a) the elements involved in the test, b) solubility results and c) observations for the test. Make sure the observations in your lab report match those that you wrote in your lab notebook during lab. The second table should be for the electronegativity tests and should include a) the elements involved in the test and b) observations and results of test. The calculation section should have the six balanced equations for the reactions being studied based on your electronegativity data. If no reaction occurred write “no reaction” as the product. The balanced equations should be in one of the following formats and either typed or written in ink.
The conclusion should be several well developed paragraphs that include the following: a discussion of the results (which was most electronegative) with support from the data section. This discussion should also include a statement with support on if the results agree with the pre-lab predictions. An explanation on how you decided which reactions occurred and which did not. A final discussion should be included on what the solubility tests indicated and using the basis for solubility if any of the results seemed irregular. Finally include a discussion on any possible errors in the experiment. Answer the Following Questions: 1) This experiment uses a qualitative colorimetric process. What is meant by this statement? 2) All of the halogens with the exception of fluorine make strong acids. Using electronegativity, explain why hydrofluoric acid is a weak acid.
|
 |
Halogen Reactions