In short, we are interested in what molecules are (molecular structure) and what they are capable of (chemical reactivity). Sometimes, this is the main motivation for a study but most often we use our findings to design molecules with interesting and useful properties. Research in photochemistry is intrinsically multidisciplinary and provides students in my group with training for their future careers in organic, physical, analytical and biochemistry both in industry and in academia. The art of making organic molecules plays important role in our projects and solid knowledge of organic synthesis is a critical foundation for every project in our group. But the real fun starts once the molecules are made and one's ability to design a system which performs better than nature is subjected to a critical test!
C1-C5 Cyclization of Enediynes: Photochemically-induced radical-anionic C1C5 cyclization of enediynes represents a new type of cycloaromatization reaction - the “cyclorearomatization” reaction which is driven by rearomatization in the vicinity of the TS. These theoretically interesting processes were discovered in our laboratory. They complement the classic Bergman cyclization of enediynes from a mechanistic (they proceed through radical-anionic rather than through a radical pathway) and a practical point of view (formation of five-membered rather than six-membered rings and abstraction of four rather than two hydrogen atoms as the result of the cycloaromatization cascade).
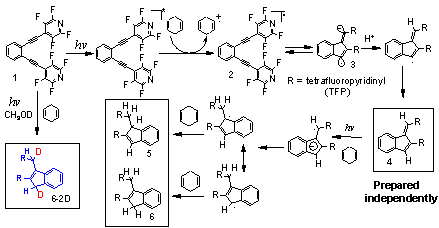
We used theoretical studies to unravel the combination of factors that renders the C1C5 cyclization more favorable than the Bergman (C1C6) pathway. Experimental work unambiguously established photoinduced electron transfer (PET) as the triggering event for the C1C5 cascade and the intermediacy of the second PET step in the indene-forming cascade. For example, two deuterium atoms are incorporated into the substituted indene product during photolysis in the presence of CH3OD. The reaction proceeds only in polar solvents and only when a suitable electron donor is present (e.g., 1,4-CHD (DGPET= -22 kcal/mol) or Et3N (DGPET= -35 kcal/mol)). We have prepared the fulvene intermediate independently through Bu3Sn radical mediated 5-exo-cyclization of enediynes and found that, unlike the stable benzene product of the Bergman cycloaromatization, fulvene product of C1C5 cyclization is indeed capable of further photoreduction to indene.
We applied this reaction for the design of efficient double-strand (ds) DNA photocleaving agents with higher DNA-damaging power and selectivity towards cancer cells and expanded our research program to the field of DNA photochemistry. A number of water-soluble enediynes equipped with DNA-recognition elements have been prepared and shown to be efficient ds-DNA photocleavers. Statistical analysis of DNA cleavage unambiguously shows that lysine-enediyne conjugates induce 100 times more ds DNA cleavage than expected from a combination of random coincident single stranded cleavages. Cytotoxicities and antitumor activities of these compounds are being investigated in collaborative studies with Mayo Clinic.
Supramolecular Chemistry: Diaryl acetylenes, in which one of the aryl groups is either a pyridine or a pyrazine, undergo efficient triplet state photocycloaddition to 1,4-cyclohexadiene with formation of 1,5-diaryl substituted homoquadricyclanes. Mechanistic and photophysical studies suggest that photocycloaddition proceeds through an electrophilic triplet excited state. Chemical and quantum yields for the cycloaddition, in general, correlate with the electron acceptor character of aryl substituents but are attenuated by photophysical factors, such as the competition between the conversion of acetylene singlet excited state into the reactive triplet excited states (intersystem crossing: ISC) and/or to the radical-anion (photoelectron transfer from the diene to the excited acetylene: PET). Dramatically enhanced ISC between p,p* S1 state and "phantom" n,p* triplet excited state (the El-Sayed rules) is likely to be important in directing reactivity to the triplet pathway.
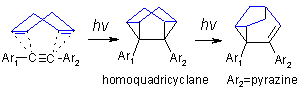
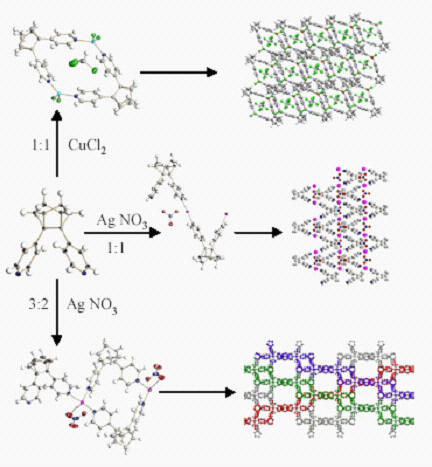
From a practical perspective, the above reactions are interesting because "capping" of the triple bond with the polycyclic framework orients the terminal aryl (4-pyridyl, 4-tetrafluoropyridyl, phenyl, etc.) groups in an almost perfect 60° angle and renders such molecules promising supramolecular building blocks suitable for the preparation of molecular rhomboids and coordination polymers.
-
Zeidan, T.; Clark, R. J.; Kovalenko, S. V.; Ghiviriga, I.; Alabugin I. V. “Triplet acetylenes as Synthetic Equivalents of 1,2-Dicarbenes. II. New Supramolecular Scaffolds from Photochemical Cycloaddition of Diarylacetylenes to 1,4-Cyclohexadienes”, Chemistry Eur. Journal, 2005, in press
-
Kovalenko, S. V.; Alabugin I. V. “Lysine-Enediyne Conjugates as Photochemically Triggered DNA Double-Strand Cleavage Agents”. Chem. Comm. 2005, 1444-1446.(in PDF)
-
Zeidan, T.; Kovalenko, S. V.; Manoharan, M.; Clark, R. J.; Ghiviriga, I.; Alabugin I. V. “Triplet Acetylenes as Synthetic Equivalents of 1,2-Dicarbenes. Phantom n,p* State Controls Reactivity in Triplet Photocycloaddition”, J. Am. Chem. Soc. 2005, 127, 4270-4285.(in PDF)
-
Peabody, S.; Breiner, B.; Kovalenko, S. V.; Patil, S.; Alabugin I. V. “Synthesis of Selectively Deuterated Fulvenes and Indenes from Enediynes” Org. Biomol. Chem. 2005, 3, 218-221.(in PDF)
-
Kovalenko, S. V.; Peabody, S.; Manoharan, M.; Clark, R. J., Alabugin I.V. “5-Exo-dig Radical Cylization of Enediynes” Org. Lett. 2004, 6, 2457-2460.(in PDF)
-
Alabugin, I. V.; Kovalenko, S. V. “C1-C5 Photochemical Cyclization of Enediynes” J. Am. Chem. Soc., 2002, 124, 9052-9053.(in PDF)
Development of New Photochemical Reactions
pH-Activated Anticancer Agents




