A. Stereoelectronic Effects.
A very important component of our research is the use of computational methods.
We are particularly interested in the influence of through-space/through-bond
orbital interactions and
stereoelectronic effects on ground-state and photochemical processes.
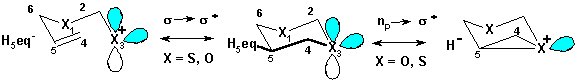
Stereoelectronic effects are ubiquitous in chemistry. Depending on the nature of interacting orbitals hyperconjugative stereoelectronic interactions can provide electron density to electron-deficient centers or withdraw it from electron-rich centers, and may stabilize incipient bonds and radical centers. These effects influence conformational equilibria (for example, anomeric effect, conformational behavior of the phosphodiester backbone in nucleic acids, conformational stability of collagens, torsion barrier in ethane and other molecules). They were found to modify reactivity, control selectivity and play important rolesin intermolecular interactions, both in ground and transition states. Even weak stereoelectronic interactions are enhanced dramatically in electronically excited, radical and ionic species.
We have identified several fundamental rules which control relative magnitudes of stereoelectronic effects. For a more detailed discussion, see:
-
Alabugin I. V.; Zeidan, T. A. Stereoelectronic Effects and General Trends in Hyperconjugative Acceptor Ability of σ Bonds. J. Am. Chem. Soc., 2002, 124, 3175-3185.(in PDF)
-
Alabugin I. V. Stereoelectronic interactions in cyclohexane, 1,3-dioxane, 1,3-oxathiane and 1,3-dithiane: W-effect, sC-X«s*C-H interactions, anomeric effect - what is really important? J. Org. Chem., 2000, 65, 3910-3919.(in PDF)
B. Improper Hydrogen Bonds.
Hydrogen bonding is the essential to many chemical and biochemical processes. A characteristic feature of H…Y hydrogen bond formation in an X-H…Y system is X-H bond lengthening with a concomitant red shift of the X-H stretching frequency. The latter, readily observed in the IR spectra, is widely regarded as the “signature of H-bonding”. However, a number of experimental and theoretical studies have reported the existence of an unusual class of “improper” or “blue-shifted” hydrogen bonds in which H-bond formation leads to X-H bond shortening and to a blue shift of the X-H IR stretching frequency. Although this effect has been reported mainly for C-H bonds, recent theoretical studies suggest that improper H-bonding is more general and can be observed for Si-H, P-H, and even N-H bonds.
We have provided a general explanation to this unusual phenomenon and predicted new types of blue-shifted H-bonds:
-
Alabugin, I. V., Manoharan, M., Peabody S., Weinhold, F. The Electronic Basis Of Improper Hydrogen Bonding: A Subtle Balance Of Hyperconjugation And Rehybridization. J. Am. Chem. Soc., 2003, 125, 5973-5987.(in PDF)
Development of New Photochemical Reactions
pH-Activated Anticancer Agents
Computational Chemistry




