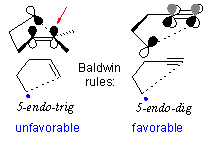
Search for the elusive 5-endo-dig radical cyclizations of carbon-centered radicals
A classic paper by Jack Baldwin (the most cited paper in the 40+ history of Chemical Communications) predicted that, unlike the 5-endo-trig closure in alkenes, the 5-endo-dig ring closure of alkynes should be favorable. Nevertheless, efficient formation of a C-C bond in a 5-endo-dig radical cyclization step remained an unachieved goal up until recently when the first example of this reaction was discovered in our lab.
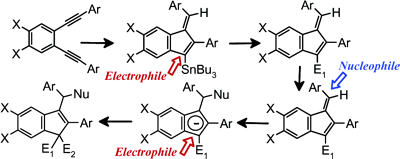
Synthesis of functionalized fulvenes and indenes
We found that Bu3Sn-mediated 5-exo-dig radical cyclization of diaryl enediynes provides a mild and efficient approach to tin-substituted fulvenes. We studied further synthetic opportunities opened by this process as well as the general factors responsible for the observed regio- and stereoselectivity. The experimental studies developed into a convenient approach to numerous substituted indenes and fulvenes which represent promising ligands for organometallic catalysis. It is known that indenyl complexes often perform better than their cyclopentadienyl analogues and that the introduction of substituents at the indenyl moiety has a dramatic influence on catalytic properties
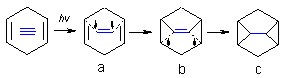
Radical cascades involved in triplet photocycloadditions of diaryl acetylenes to cyclic 1,4-dienes
Diaryl acetylenes, in which one of the aryl groups is either a pyridine or a pyrazine, undergo efficient triplet state photocycloaddition to 1,4-cyclohexadiene with formation of 1,5-diaryl substituted tetracyclo[3.3.0.02,8.04,6]octanes (homoquadricyclanes). The mechanism is complex but the topological scheme for this transformation can be described by the simple scheme given below. Photochemical excitation transforms an alkyne (shown in blue) into an electrophilic diradical which can attack a p-bond of an alkene. This attack generates a new radical which "comes back" by making a bond to the same carbon of alkyne which has been involved into the initial C-C bond formation.
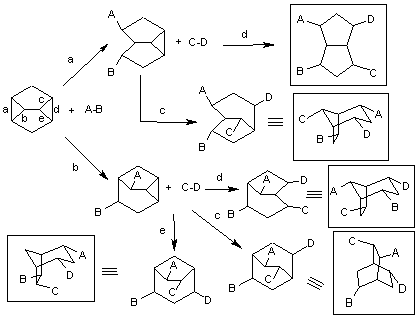
The next challenge is to utilize homoquadricyclanes for selective organic transformations shown below:
Development of New Photochemical Reactions
pH-Activated Anticancer Agents
Design and discovery of new radical transformations




